Review | Open Access
Research progress of perioperative immunotherapy for locally advanced gastric cancer
Shanbo Ma1, *, Wei Zhang2, *, Xiaodi Guo1, Yuhan Chen3, Zhiyong Zhao4, Hongbo Jiang5
1The College of Life Sciences, Northwest University, Xi’an City, P.R. China.
2Department of Liver Disease, Daxing Hospital, Xi’an City, P.R. China.
3Drug Clinical Trial Institution of The Second Affiliated Hospital of Shaanxi University of Traditional Chinese Medicine, Xianyang City, P.R. China.
4Shenzhen Hospital, Beijing University of Chinese Medicine, Shenzhen City, P.R. China.
5Shaanxi Province Forest Industry Worker Hospital, Xi’an City, P.R. China.
*: These authors contributed equally.
Correspondence: Zhiyong Zhao (Shenzhen Hospital, Beijing University of Chinese Medicine, Shenzhen 51800, P.R. China; Email: China.zzy695779347@163.com.) and Hongbo Jiang (Shaanxi Province Forest Industry Worker Hospital, Xi’an 710300, P.R. China; Email: China.330539192@qq.com).
Asia-Pacific Journal of Oncology 2024, 5: 1-8. https://doi.org/10.32948/ajo.2024.03.01
Received: 22 Feb 2024 | Accepted: 07 Mar 2024 | Published online: 08 Mar 2024
Key words locally advanced gastric cancer, perioperative period, immunotherapy, neoadjuvant therapy, conversion therapy, tumor vaccine, cell therapy
ICIs monotherapy
In recent years, many studies have investigated the efficacy of ICI monotherapy in patients with untreated gastric or gastroesophageal junction cancer.
I. Pabolizumab monotherapy. In KEYMAT-062's Phase III trial [21], it was found that for patients with a combined positive score (CPS) of PD-L1 ≥1, the median overall survival in the Pabolizumab group was no worse than that of chemotherapy alone (cisplatin + fluorouracil; Capecitabine group (10.6 months vs. 11.1 months), but also did not show a significant advantage; For patients with PD-L1 CPS≥10, the median overall survival was 17.4 and 10.8 months, respectively, and the abolition monotherapy group showed numerical superiority: but, this difference was not statistically compared. The incidence of grade 3 to 5 adverse events was found to be 17% and 69% in the Pabolizon monotherapy group and chemotherapy alone group, respectively, suggesting that the Pabolizon monotherapy group was safer and better tolerated. In the KEYMAT-063 Phase III trial [22], it was found that the incidence of grade 3 to 5 adverse reactions in PD-L1 positive (CPS≥1) advanced gastric or gastroesophageal junction adenocarcinoma patients was 11% and 64%, respectively, and Pabolizil was more reliable than paclitaxel in terms of safety. Pabolizil did not show any advantage over paclitaxel in terms of median overall survival and median progression-free survival [8.0 months vs. 8.0 months, hazard ratio (HR) and 95% confidence interval (CI) of 0.99 (0.63, 0.95); 2.0 months vs. 4.0 months, HR (95%CI) was 1.62 (1.04, 2.52)]. Other second-line treatments, such as pabolizon in KEYNOTE-061 Phase III trials [23], did not extend overall survival in patients with PD-L1-positive advanced gastric or gastroesophageal junction adenocarcinoma. The above trial results suggest that ICI monotherapy Pabolizon has limited efficacy in the treatment of PD-L1-positive advanced gastric or gastroesophageal junction carcinoma patients, and stratification of PD-L1-positive patients may be needed to explore the efficacy of immune monotherapy or further explore treatment options suitable for different patients.
II. Study on the treatment of gastric or gastroesophageal junction carcinoma with Averumab. In the JAVELIN Gastric 100 trial [24], avilumab was used as maintenance therapy for advanced gastric cancer or gastroesophageal junction adenocarcinoma patients, and compared with chemotherapy alone, there was no advantage in median overall survival in the avilumab group (10.4 months versus 10.9 months). In PD-L1 positive (CPS≥1) patients, the median overall survival was 14.9 and 11.6 months, respectively (P=0.635), and averumab did not show better clinical outcomes, suggesting that when treating advanced gastric cancer or gastroesophageal junction adenocarcinoma, Avilumab maintenance therapy did not increase clinical benefit in all patients or PD-L1-positive patients, and its efficacy as a monotherapy in patients with advanced gastric cancer needs to be validated in further trials. Other PD-1 inhibitors [25], such as triplizumab in the treatment of advanced gastric cancer, found that the results of treatment with triplizumab monotherapy and its combination with XELOX (capecitabine and oxaliplatin) in patients with refractory gastric cancer showed that the objective response rates of the 2 groups were 12.1% and 66.7%, respectively. The disease control rate was 39.7% and 88.9%, the incidence of treatment-related adverse reactions was 77.6% and 94.4%, and the incidence of ≥ grade 3 treatment-related adverse reactions was 22.4% and 38.9%, respectively. PD-1 monoclonal antibody Tripril has a controllable safety profile and promising antitumor activity in the treatment of advanced gastric cancer and is more effective when combined with XELOX. In the above clinical trials, only KEYNOTE-062 showed a numerical advantage over chemotherapy in terms of median overall survival when Pabolizumab was used in the treatment of PD-L1 CPS≥10 patients, while the other trials showed efficacy equal to or no worse than chemotherapy in the treatment of PD-L1 positive patients. Although ICIs alone have no significant advantage over chemotherapy alone in terms of efficacy, they show good anti-tumor activity in combination with chemotherapy. The advantage of ICI monotherapy is that the efficacy of ICIs is not inferior to that of chemotherapy while the incidence of adverse reactions is low. Further studies can be conducted in the future to screen out ICIS monotherapy patients with greater benefits in terms of economy and efficacy.
ICIs combined with chemical drugs
The efficacy of ICI monotherapy in the treatment of advanced gastric cancer is not superior to chemotherapy, but a large number of studies have shown that ICI combined chemotherapy has better clinical outcomes than chemotherapy alone.
I. Parbolizol in combination with chemotherapy In the KEYKEYNOTE 059 trial [26], gastric cancer patients with PD-1 CPS≥1 were divided into Pbolizumab combined chemotherapy (combination group) and pbolizumab monotherapy (pbolizumab monotherapy group), and the results showed that the objective response rate of the combination group and Pbolizumab monotherapy group was 60.0% [95%CI (38.7%), 78.9%)] and 25.8% [95%CI (11.9%, 44.6%)], the combination showed better efficacy than the monotherapy, but parbolizul monotherapy showed a lower incidence of grade 3 to 5 adverse events in terms of safety (76.0% vs. 22.6% in the 2 groups, respectively).
II. Nebuliumab in combination with chemotherapy In the ATTRACTION 4 trial [27], the difference in efficacy between the nabriliumab combined chemotherapy group (nabriliu group) and the placebo combined chemotherapy group (placebo group) was investigated, and the Nabriliu group did not show a significant advantage in terms of median overall survival (17.45 months vs. 17.15 months, P=0.26). However, it showed a significant benefit in median progression-free survival (10.45 months vs. 8.34 months, P<0.001), with a higher response rate and longer duration of response; also, in the CheckMate-649 Phase III trial [28], it was found that naboliuzumab combined with chemotherapy showed longer progression-free survival, longer duration of response, and a higher response rate in all randomized patients compared to chemotherapy alone. The results of the above two clinical trials all showed that nebuliumab combined with chemotherapy as first-line treatment had positive effects on human epidermal growth factor receptor type 2, Patients with HER2 negative, unresectable advanced or recurrent gastric cancer or gastroesophageal junction cancer may benefit.
III. Tirellizumab in combination with chemotherapy. In a Phase II NCT03469557 trial [29], the objective response rate and disease control rate of tirellizumab combined with chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma and gastric or gastroesophageal junction adenocarcinoma were 46.7% and 80%, respectively. The most common adverse event was anemia. This trial demonstrated that tirellizumab combined with chemotherapy has good efficacy and a manageable safety profile in advanced esophageal squamous cell carcinoma and gastric or gastroesophageal junction adenocarcinoma.
IV. Sindilizumab in combination with chemotherapy. Wu et al. [30] retrospectively analyzed the efficacy and safety of PD-1 inhibitor Xindilizumab combined with XELOX in patients with advanced gastric cancer, and the results showed that 1 out of 10 patients achieved clinical complete remission, 6 patients achieved clinical partial remission, and 3 patients had clinically stable disease without disease progression. None of the 10 patients had grade 3 or higher adverse reactions in terms of safety. The results suggested that the PD-1 inhibitor Sindilizumab combined with the XELOX regimen was effective for advanced gastric cancer. However, this study also pointed out deficiencies, that is, a small number of patients were included. In general, the results of most existing studies using immunotherapy combined with chemotherapy are encouraging. Immunotherapy combined with chemotherapy is better than chemotherapy alone in the treatment of advanced gastric cancer patients with PD-L1 CPS≥1, and the treatment of immunotherapy combined with chemotherapy can significantly improve the survival of patients. Only in the Phase III KEYNOTE-062 trial [21], it was found that pabolizumab combined with chemotherapy as the first-line treatment for patients with advanced gastric cancer with PD-L1 CPS≥1 or higher did not show better overall survival or progression-free survival than chemotherapy alone. This difference is worthy of further exploration for its reasons.
Immunotherapy combined with chemoradiotherapy
Radiotherapy can increase the efficacy of immune drugs. After radiotherapy, the expression of the major histocompatibility complex (MHC) on the tumor surface is upregulated, which enhances the recognition of tumor cells by cytotoxic T cells. At the same time, radiotherapy promotes the release of various cytokines, induces the activation of immune cells, changes the immune characteristics of tumors, and enhances the sensitivity of ICIs [31, 32]. In the perioperative treatment of locally advanced gastric cancer, a Phase II trial (NCT02730546) investigated the safety and efficacy of preoperative use of pabolizumab in combination with concurrent chemoradiotherapy in patients with gastroesophageal junction cancer. Seven patients (22.6%) achieved PCR but did not meet the prespecified primary endpoint [33]. However, in the ⅡNeoPLANET(NCT03631615) trial, the application of neoadjuvant carrellizumab combined with chemoradiotherapy in locally advanced adenocarcinoma at the gastric or gastroesophageal junction was studied. The primary endpoint was the pathologic complete response (PCR rate was 33.3%(95%CI 18.6-51.0), which reached the pre-specified endpoint. The resection rates of TpCR, MPR, and R0 were 33.3%, 44.4%, and 91.7%, respectively. 77 patients (8.0%) achieved ypN0. The 2-year progression-free survival and OS rates were 66.9% and 76.1%, respectively [34]. Neoadjuvant carrilizumab combined with chemoradiotherapy has demonstrated a promising pathological response in patients with locally advanced gastric adenocarcinoma. In addition, in a multicenter, single-arm Phase II trial (ChiCTR1900024428), patients with locally advanced gastric cancer/gastroesophageal junctional adenocarcinoma received preoperative immunotherapy combined with chemoradiotherapy (anti-PD-1, S-1, and NAB-paclitaxel), followed by 3 cycles of adjuvant sindiglimab and chemotherapy. The primary endpoint was pCR. median disease-free survival (mDFS) and EFS were 17.0 (95%CI: 11.1-20.9) months and 21.1 (95%CI: 11.1-20.9) months, respectively. In 14.7 to 26.1 months, the median OS was not reached, and the 1-year OS rate was 92.6%(95%CI: 50.1 to 99.5) [35]. It is suggested that Sindilizumab combined with concurrent chemoradiotherapy has a promising effect in the perioperative treatment of locally advanced gastric cancer/gastroesophageal junction adenocarcinoma. Based on the results of the CROSS study, chemoradiotherapy has become the standard neoadjuvant therapy for patients with resectable esophageal cancer or gastroesophageal junction adenocarcinoma [36]. The PERFECT(NCT03087864) trial demonstrated the efficacy and safety of immunization combined with concurrent chemoradiotherapy in esophageal adenocarcinoma [37], but did not achieve the expected effect in the perioperative trial NCT02730546 in gastric cancer. Neo-PLANET and ChiCTR1900024428 studies demonstrated the effectiveness of immunotherapy combined with concurrent chemoradiotherapy in the perioperative treatment of advanced gastric cancer. Neoadjuvant PD-1 inhibitor combined with concurrent chemoradiotherapy may delay the progression of gastric cancer/gastroesophageal conjunctional adenocarcinoma. However, these two trials were single-arm designs with small sample sizes, and it is necessary to conduct a large-scale randomized controlled trial to further verify the efficacy of PD-1 combined with concurrent chemoradiotherapy in the perioperative treatment of locally advanced gastric adenocarcinoma. The application of immunization combined with concurrent chemoradiotherapy in perioperative gastric cancer is still under investigation.
ICIs combined with targeted therapy + chemotherapy
Targeted drugs themselves can inhibit the growth of tumor cells and, at the same time up-regulate the expression of PD-1 and its ligand, induce the expression of tumor-infiltrating lymphocytes, and up-regulate MHC Ⅱ molecules [38-41]. At this time, the application of PD-L1 inhibitors can enhance the killing effect of immune cells. In several phase I/II studies, antiangiogenic agents have been shown to reprogram TME to reverse immunosuppression to an inflammatory state, working synergically with ICIs to promote a local immune response [42-44]. A single-arm Phase II exploratory trial (NCT03878472) evaluated the efficacy of neoadjuvant/translational therapy in combination with ICIs (carralizumab), anti-angiogenic agents (Apatinib), and chemotherapy (S-1 ± oxaliplatin) in the treatment of cT4a/bN+ gastric cancer, the primary endpoint being pathological response and its potential biomarkers. PCR and MPR were 15.8% and 26.3%, respectively. Pathological reactions were significantly correlated with microsatellite instability, PD-L1 expression, and tumor mutation load [45]. However, it is important to note that the relatively short duration of treatment received by the patients in this study, including the number of treatment cycles and the interval between the last apatinib and surgery, may not be sufficient to achieve a fully materialized immune response, especially for patients with cT4 stage gastric cancer, and extending the duration of treatment may improve outcomes. In addition, the Dragon-Ⅳ/ AAHEAD G208 study showed that the postoperative pCR rate of patients with advanced gastric cancer undergoing surgery after neoadjuvant therapy with Apatinib + carrilizumab +SOX regimen was 18.3%, which was significantly higher than 5.0% in the SOX regimen chemotherapy group [46]. This result confirmed the feasibility of the combination therapy mechanism of targeting, immunization, and chemotherapy, enriched the perioperative treatment options for patients with gastric cancer and brought hope for their long-term survival benefits. On the other hand, human epidermal growth factor receptor 2 (HER2) positive gastric cancer can be resected with perioperative chemotherapy combined with Tirelli.
The results of the study on therapeutic efficacy and safety of zumab and trastuzumab (NCT04819971) were presented at the ASCO meeting in 2023, and the pCR rate reached more than 58%, indicating the synergistic effect of the combination of anti-HER2 therapy and immunotherapy. The combination of anti-HER2 therapy and immunotherapy represented by Tirellizumab improved the efficacy of patients with HER2-positive gastric cancer. The TAOS-3B study (NCT05223088) explores the efficacy and safety of tirellizumab + Apatinib +SOX in the perioperative treatment of locally advanced gastric cancer, with promising results. Although the single-arm design of the combination therapy could not distinguish the relative contribution of each component (immunotherapy, targeted therapy, and chemotherapy) to the therapeutic effect and immune activation, in general, the combination of immune targeted therapy and chemotherapy achieved good efficacy in the perioperative treatment of locally advanced gastric cancer and enriched the treatment options for perioperative treatment of locally advanced gastric cancer. In patients with HER2-positive gastric cancer, the combination of anti-HER2 therapy and immune drugs will also create a new treatment model.
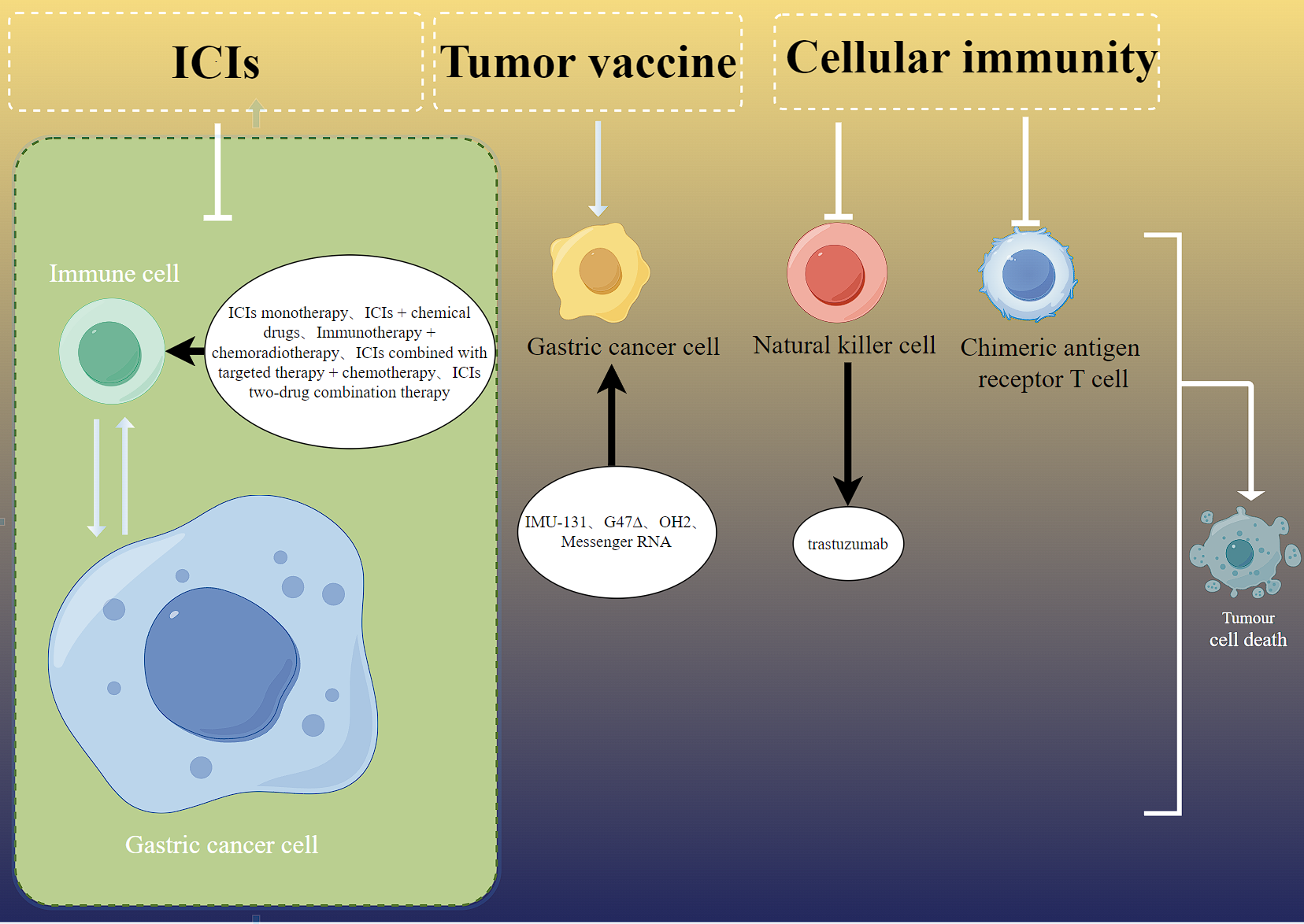 Figure 1. Perioperative immunotherapy for locally advanced gastric cancer. ICIs can block programmed cell death protein 1 (PD-1) by blocking programmed death-ligand 1 (PD-L1); The co-inhibitory signaling pathway mediated by immune checkpoints such as LAG-3 inhibits the killing of tumor killer cells on tumor cells and reactivates the immune response of human cells. ICIs: immune checkpoint inhibitors; PD-1: programmed cell death protein 1; PD-L1: programmed death-ligand 1; CTLA-4: cytotoxic T-lymphocyte associated antigen 4; OH2: a genetically engineered oncolytic herpes simplex virus type 2; G47∆: a kind of Oncolytic virus.
Figure 1. Perioperative immunotherapy for locally advanced gastric cancer. ICIs can block programmed cell death protein 1 (PD-1) by blocking programmed death-ligand 1 (PD-L1); The co-inhibitory signaling pathway mediated by immune checkpoints such as LAG-3 inhibits the killing of tumor killer cells on tumor cells and reactivates the immune response of human cells. ICIs: immune checkpoint inhibitors; PD-1: programmed cell death protein 1; PD-L1: programmed death-ligand 1; CTLA-4: cytotoxic T-lymphocyte associated antigen 4; OH2: a genetically engineered oncolytic herpes simplex virus type 2; G47∆: a kind of Oncolytic virus.
None.
Availability of data and materials
Data and materials are available on request from the authors.
Ethical policy
Not applicable.
Author contributions
SBM and WZ conceptualized, designed, conducted research, and wrote the first draft. XDG, AND YHC contributed to the revision and figure production, ZYZ amd JBJ provided supervision and revision of the draft.
Competing interests
The authors have no conflicts of interest regarding the publication of this paper.
Funding
None.
- Sung H, Ferlay J, Siegel RL: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71(3): 209-249.
- Siegel RL, Miller KD: Cancer Statistics, 2021. CA Cancer J Clin 2021, 71(1):7-33.
- Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F: Gastric cancer. Lancet 2020, 396(10251): 635-648.
- Zheng RS, Zhang SW, Sun KX, Chen R, Wang SM, Li L, Zeng HM, Wei WW, He J: [Cancer statistics in China, 2016]. Zhonghua Zhong Liu Za Zhi 2023, 45(3): 212-220.
- Xu RH, Zhang Y, Pan H, Feng J, Zhang T, Liu T, Qin Y, Qin S, Yin X, Liu B, et al: Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): a randomized, multicentre, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2021, 6(12): 1015-1024.
- Ford AC, Yuan Y, Moayyedi P: Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut 2020, 69(12): 2113-2121.
- Tramacere I, Pelucchi C, Bagnardi V, Rota M, Scotti L, Islami F, Corrao G, Boffetta P, La Vecchia C, Negri E: A meta-analysis on alcohol drinking and esophageal and gastric cardia adenocarcinoma risk. Ann Oncol 2012, 23(2): 287-297.
- Lu L, Mullins CS: A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond) 2021, 41(11): 1137-1151.
- Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC: Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022, 33(10): 1005-1020.
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ et al: Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006, 355(1): 11-20.
- Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B et al: Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011, 29(13): 1715-1721.
- In H, Solsky I, Palis B, Langdon-Embry M, Ajani J, Sano T: Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann Surg Oncol 2017, 24(12): 3683-3691.
- Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X et al: Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol 2013, 14(12): e535-547.
- Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, Yu J, Bu Z, Chen L, Du Y et al: Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomized controlled trial. Lancet Oncol 2021, 22(8): 1081-1092.
- Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, Tanabe K, Ohdan H: The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer 2018, 21(2): 315-323.
- Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z, Fassan M, Rugge M, Valeri N, Okines A et al: Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol 2017, 3(9): 1197-1203.
- Mariotto AB, Noone AM, Howlader N, Cho H, Keel GE, Garshell J, Woloshin S, Schwartz LM: Cancer survival: an overview of measures, uses, and interpretation. J Natl Cancer Inst Monogr 2014, 2014(49): 145-186.
- Olson DJ, Eroglu Z: Pembrolizumab Plus Ipilimumab Following Anti-PD-1/L1 Failure in Melanoma. J Clin Oncol 2021, 39(24):2647-2655.
- Zhang F, Guo W, Zhou B, Wang S, Li N, Qiu B, Lv F, Zhao L, Li J, Shao K et al: Three-Year Follow-Up of Neoadjuvant Programmed Cell Death Protein-1 Inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2022, 17(7): 909-920.
- Yu Y, Ma X, Zhang Y, Zhang Y, Ying J, Zhang W, Zhong Q, Zhou A, Zeng Y: Changes in Expression of Multiple Checkpoint Molecules and Infiltration of Tumor Immune Cells after Neoadjuvant Chemotherapy in Gastric Cancer. J Cancer 2019, 10(12): 2754-2763.
- Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J et al: Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol 2020, 6(10): 1571-1580.
- Chung HC, Kang YK, Chen Z, Bai Y, Wan Ishak WZ, Shim BY, Park YL, Koo DH, Lu J, Xu J et al: Pembrolizumab versus paclitaxel for previously treated advanced gastric or gastroesophageal junction cancer (KEYNOTE-063): A randomized, open-label, phase 3 trial in Asian patients. Cancer 2022, 128(5): 995-1003.
- Fuchs CS, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic C, Chung HC et al: Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer 2022, 25(1): 197-206.
- Moehler M, Dvorkin M, Boku N: Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J Clin Oncol 2021, 39(9): 966-977.
- Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, Yuan XL, Chen Y, Yang SJ, Shi JH, et al: Safety, efficacy, and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol 2019, 30(9): 1479-1486.
- Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI et al: Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 2019, 22(4): 828-837.
- Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S et al: Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022, 23(2): 234-247.
- Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A et al: First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021, 398(10294): 27-40.
- Xu J, Bai Y, Xu N, Li E, Wang B, Wang J, Li X, Wang X, Yuan X: Tislelizumab Plus Chemotherapy as First-line Treatment for Advanced Esophageal Squamous Cell Carcinoma and Gastric/Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res 2020, 26(17): 4542-4550.
- Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, Liu X: Application of PD-1 Blockade in Cancer Immunotherapy. Comput Struct Biotechnol J 2019, 17: 661-674.
- Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC: Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005, 11(2 Pt 1): 728-734.
- Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S: Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009, 15(17): 5379-5388.
- Zhu M, Chen C, Foster NR, Hartley C, Mounajjed T, Salomao MA, Fruth BF, Beamer SE, Kim Y, Harrington SM et al: Pembrolizumab in Combination with Neoadjuvant Chemoradiotherapy for Patients with Resectable Adenocarcinoma of the Gastroesophageal Junction. Clin Cancer Res 2022, 28(14): 3021-3031.
- Tang Z, Wang Y, Liu D, Wang X: The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat Commun 2022, 13(1): 6807.
- Wei J, Lu X, Liu Q, Fu Y: Neoadjuvant sintilimab in combination with concurrent chemoradiotherapy for locally advanced gastric or gastroesophageal junction adenocarcinoma: a single-arm phase 2 trial. Nat Commun 2023, 14(1): 4904.
- van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ et al: Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012, 366(22): 2074-2084.
- van den Ende T, de Clercq NC, van Berge Henegouwen MI: Neoadjuvant Chemoradiotherapy Combined with Atezolizumab for Resectable Esophageal Adenocarcinoma: A Single-arm Phase II Feasibility Trial (PERFECT). Clin Cancer Res 2021, 27(12): 3351-3359.
- Chaganty BKR, Qiu S, Gest A, Lu Y, Ivan C, Calin GA, Weiner LM, Fan Z: Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion. Cancer Lett 2018, 430: 47-56.
- Varadan V, Gilmore H, Miskimen KL, Tuck D, Parsai S, Awadallah A, Krop IE, Winer EP, Bossuyt V, Somlo G et al: Immune Signatures Following Single Dose Trastuzumab Predict Pathologic Response to PreoperativeTrastuzumab and Chemotherapy in HER2-Positive Early Breast Cancer. Clin Cancer Res 2016, 22(13): 3249-3259.
- Triulzi T, Regondi V, De Cecco L, Cappelletti MR, Di Modica M, Paolini B, Lollini PL, Di Cosimo S, Sfondrini L, Generali D et al: Early immune modulation by single-agent trastuzumab as a marker of trastuzumab benefit. Br J Cancer 2018, 119(12): 1487-1494.
- Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, Teng MW, Smyth MJ: Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A 2011, 108(17): 7142-7147.
- Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, Mikamoto Y, Shima H, Fujishiro N, Higuchi T et al: Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol 2020, 21(8): 1057-1065.
- Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y et al: Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol 2020, 38(18): 2053-2061.
- Peng Z, Wei J, Wang F, Ying J, Deng Y, Gu K, Cheng Y, Yuan X, Xiao J, Tai Y et al: Camrelizumab Combined with Chemotherapy Followed by Camrelizumab plus Apatinib as First-line Therapy for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res 2021, 27(11): 3069-3078.
- Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, Shi D, Yu D: Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun 2023, 14(1): 8.
- Janjigian YY, Van Cutsem E, Muro K, Wainberg Z, Al-Batran SE, Hyung WJ, Molena D, Marcovitz M, Ruscica D, Robbins SH et al: MATTERHORN: phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future Oncol 2022, 18(20): 2465-2473.
- Somaiah N, Conley AP, Parra ER, Lin H, Amini B, Solis Soto L, Salazar R, Barreto C, Chen H, Gite S et al: Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: a single-center phase 2 trial. Lancet Oncol 2022, 23(9): 1156-1166.
- Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH et al: Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021, 22(1): 51-65.
- Shitara K, Ajani JA: Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022, 603(7903): 942-948.
- Kelly RJ, Lee J: Safety and Efficacy of Durvalumab and Tremelimumab Alone or in Combination in Patients with Advanced Gastric and Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res 2020, 26(4): 846-854.
- Evrard C, Louvet C, Hajbi FE, Fiore FD, Malicot KL, Aparicio T, Bouché O, Laurent-Puig P, Bibeau F, Lecomte T et al: PRODIGE 59-DURIGAST trial: A randomised phase II study evaluating FOLFIRI + Durvalumab ± Tremelimumab in second-line of patients with advanced gastric cancer. Dig Liver Dis 2021, 53(4): 420-426.
- Wiedermann U, Garner-Spitzer E, Chao Y, Maglakelidze M, Bulat I, Dechaphunkul A, Arpornwirat W, Charoentum C, Yen CJ, Yau TC et al: Clinical and Immunologic Responses to a B-Cell Epitope Vaccine in Patients with HER2/neu-Overexpressing Advanced Gastric Cancer-Results from Phase Ib Trial IMU.ACS.001. Clin Cancer Res 2021, 27(13): 3649-3660.
- Fucikova J, Hensler M, Kasikova L, Lanickova T, Pasulka J, Rakova J, Drozenova J, Fredriksen T, Hraska M, Hrnciarova T: An Autologous Dendritic Cell Vaccine Promotes Anticancer Immunity in Patients with Ovarian Cancer with Low Mutational Burden and Cold Tumors. Clin Cancer Res 2022, 28(14): 3053-3065.
- Zhang W, Lu X, Cui P, Piao C, Xiao M, Liu X, Wang Y, Wu X, Liu J, Yang L: Phase I/II clinical trial of a Wilms' tumor 1-targeted dendritic cell vaccination-based immunotherapy in patients with advanced cancer.Cancer Immunol Immunother 2019, 68(1): 121-130.
- Todo T, Ito H, Ino Y: Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial. Nat Med 2022, 28(8): 1630-1639.
- Zhang B, Huang J: Intratumoral OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: a multicenter, phase I/II clinical trial.J Immunother Cancer 2021, 9(4): e002224.
- Rojas LA, Sethna Z, Soares KC: Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618(7963): 144-150.
- Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR et al: Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019, 380(1): 45-56.
- Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, Ghobadi A, Rapoport AP, McGuirk J, Pagel JM et al: Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med 2022, 386(7): 640-654.
- Qi C, Gong J, Li J, Liu D, Qin Y, Ge S, Zhang M, Peng Z, Zhou J, Cao Y et al: Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med 2022, 28(6): 1189-1198.
- Heczey A, Xu X: Anti-GD2 CAR-NKT cells in relapsed or refractory neuroblastoma: updated phase 1 trial interim results. Nat Med 2023, 29(6): 1379-1388.
- Lee SC, Shimasaki N, Lim JSJ, Wong A, Yadav K: Phase I Trial of Expanded, Activated Autologous NK-cell Infusions with Trastuzumab in Patients with HER2-positive Cancers. Clin Cancer Res 2020, 26(17): 4494-4502.
Cite this article: Ma SB, Zhang W, Guo XD, Chen YH, Zhao ZY, Jiang HB: Research progress of perioperative immunotherapy for locally advanced gastric cancer. Asia Pac J Oncol 2024, 5: 1-8. https://doi.org/10.32948/ajo.2024.03.01
Asia-Pacific Journal of Oncology
print ISSN: 2708-7980, online ISSN: 2708-7999
 Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript