Research Article | Open Access
An invitro evaluation of the cytotoxicity and p53-mediated apoptotic effect of ocimum sanctum leaves hexane extract on human oral cancer cell lines
VR Naghul1, Jerrine Joseph2, Mary Shamya Arokiarajan2, V. Ramesh Kumar11Department of Biotechnology, Sathyabama Institute of Science & Technology, Chennai, Tamil Nadu, India.
2Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu, India.
Correspondence: Jerrine Joseph (Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai-600119, Tamil Nadu, India; E-mail: jerrine.jj@gmail.com).
Asia-Pacific Journal of Oncology 2022, 3: 1-9. https://doi.org/10.32948/ajo.2022.12.24
Received: 10 Dec 2022 | Accepted: 24 Dec 2022 | Published online: 27 Dec 2022
Methods Extract preparation using soxhlet apparatus, DPPH assay for antioxidant activity, Anticancer activity and western blotting for p53 expression.
Results The obtained results showed that the methanol and hexane extracts of Ocimum sanctum (Tulsi) showed better activity in DPPH assay when compared with the rest. In anticancer activity against KB cell line and cytotoxicity activity in L132 cell line which was carried out by MTT assay; Ocimum sanctum (Tulsi) Hexane extract showed IC50 value of 74.39 for KB cell line and IC50 value of 431.39 for L132 cell line. The expression of p53 gene in KB cell line showed increased level of p53 denotes its upregulation has a cascade effect on regulation of apoptosis.
Conclusion Thus substantiating the claim that it has evidenced anti oral cancer potential, which is promising but needs additional wet lab validations and characterization to isolate the phyto molecule and qualify it as a potential lead.This promising lead could be further taken up for bio activity studies of the purified phytomolecules from the plant for in vivo testing as a potential safe herbal based alternative therapeutics for oral cancer.
Key words anti-proliferative activity, ocimum sanctum, KB mouth cell line, phytomolecules
Leaves of Azadirachta indica (Neem), Mangifera indica (Mango), Ocimum sanctum (Tulsi), Carica papaya (Papaya), and Senna auriculate (Avaram) were collected from Perambur, Chennai surrounding area and validated by a Botanist. Samples were washed well with running tap water followed by distilled water to remove the impurities or salt content and shade dried.
Preparation of plant extract
To extract the Phyto molecules from plants, different solvents were used. 20g of each plant material was weighed and dissolved in 200ml of solvents (1:10) methanol and hexane were used. The extraction was carried out for 24 hr at 50℃ using Soxhlet apparatus. The extracted solution was evaporated using Rotary evaporator (Bio-rad) and then dried in Hot air oven. Once dried, the plant residues were kept in sterile bottles under refrigerated conditions for further use.
Antioxidant Assay DPPH assay
The free radical scavenging capacity of the plant extract was measured based on the method delineate by Brand-Williams [32] with slight modification. It is based on electron-transfer that produces a violet solution in methanol. This free radical, which is stable at room temperature, is reduced to colorless methanol solution in the presence of an antioxidant molecule [33]. 0.1 mM DPPH (Himedia) solution was mixed with 1ml of plant extract solution of varying concentration (10, 100, 500, 1000μg/ml) tubes was incubated at room temperature for 30 minutes in dark. Then the absorbance was measured using a UV-Vis spectrophotometer at 517nm. Gallic acid (Himedia) was used as the standard [34]. The capability of scavenging the DPPH radical was calculated by using the formula:
DPPH scavenging effect/activity
(%inhibition or %scavenging) = {(A0-A1)/A0)x100}
Cell culture
The L132 and KB cell lines purchased from the NCCS Pune, India. The cells were maintained in Minimal Essential Media supplemented with 10% FBS, penicillin (100 U/ml) and streptomycin (100µg/ml) in a 5% CO2 at 37℃.
Anticancer and cytotoxicity studies
Microculture tetrazolium technique colorimetric assay (MTT assay measures cell viability based on the generation of reducing equivalents in metabolic active cells [35]. The L132 and KB Cell lines (NCCS, Pune) were seeded at 5000 cells/well in 96-well plates and both were incubated for 48h at various concentrations of the sample (1, 10, 30, 100, 300, 1000 µg/ml) for 24h incubation. Then the sample was placed in a new medium containing 50µl of MTT solution (5mg/ml), to each well incubated for 4h and DMSO was added. DMSO used as a negative control for 48 h. The viable cells were determined by the absorbance at 570nm by microplate reader it was calculated by using the formula:
% Viability = {(A570 of treated cells)/(A570 of controlled cells) x100} / {% Cytotoxicity =100-%Viability}
Phytochemical analysis (qualitative)
To detect the presence of following bio molecules by standard qualitative phytochemical procedures [36-37]. Test for alkaloids using Mayer’s reagent (Himedia), for tannins using ferric chloride, flavonoids using ammonia, for glycosides with chloroform and 10% ammonium solution. Saponins test done by distilled water with vigorous shaking [38], presence of terpenoid confirmed with chloroform and sulfuric acid [39]. Steroids, glycoside, phenols are also tested with standard procedures.
Thin layer chromatography
Thin layer chromatography sheets (Himedia) uses the same principles as extraction to accomplish the separation and purification of compounds. Toluene: ethyl acetate: formic acid (36:12:5) [40] is used as a mobile phase in order to separate phenolic compounds from the plant extract. The plant extract was dissolved in their particular solvent and spotted in the TLC sheet and placed in the solvent system chamber. Then they were observed for the color of the band and their fluorescence under visible light, UV-254nm and UV-366nm. For visualization iodine vapor were used. The retention factor or Rf is defined as the distance travelled by the compound divided by the distance travelled by the solvent.
Western blotting
The cells were treated with different concentration of drugs for 48h. Cells were lysed with Triple detergent lysis buffer (Electrophorosis grade) contain 50M Tris-Hcl,150 mM Nacl,0.1% SDS,100ug/ml PMSF,1ug/ml Aprorinin,1% NP-40 with pH 8.3. Add 10-20ul of sample to same amount of 2X loading buffer (1:1), mix well, and include Protein Marker boil it for 5-10 min. Quantified proteins were performed in SDS-PAGE. The separated proteins were transferred to nitrocellulose membranes. Membrane was washed with Western Transfer buffer (10% methanol, 24mM Tris, 194mM glycine, blocked with 5-10% non-fat dried milk in PBS for 1 hour or overnight at 4℃ with shaking. Wash in PBS with 0.1% Tween 20 for three times. The membranes then incubated with Primary Antibody in 1%BSA in PBS for 1 hour at room temperature or overnight at 4℃ and Wash in PBS with 0.1%Tween 20 for three times. Again the membranes were incubated with secondary antibody (peroxidase- conjugated goat anti-mouse IgG, etc). After Washing with PBS and 0.1%Tween 20, they were developed with DAB chromogenic detection method and scanned.
Statistical analysis
All in vitro assay data signify the mean ± standard deviation of triplicates and IC50 was calculated by using one way analysis of variances (ANOVA).
Crude extract has been prepared from the leaves of Azadirachta indica (neem), Mangifera indica (Mango), Ocimum sanctum (Tulsi), Carica papaya (Papaya), and Senna auriculate (Avaram) in Figure 1. Antioxidant activity of the extracts was accessed by adopting DPPH assay. In this method the best antioxidant activity was found in Tulsi Hexane (84 %) and Tulsi Methanol (83.2%) extract. Whereas the other leaf extracts such as neem hexane 79.2 % and methanol 81.5%; papaya hexane 83.1% and methanol 76.2%; avaram hexane 80.6% and methanol 75.2%, and the values were represented in Figure 2 (A&B). Based on their antioxidant property tulsi were taken to test against the oral cancer cell line. The hexane extract of O. sanctum leaves exhibited significant cytotoxic effect against L132 cell line. They were compared with control group which was untreated L132 cell line. For Cell viability test cell line was treated with both the extracts (methanol and hexane) with 6 different concentrations. The optimized IC50 values of hexane extract is 431.39 μg/ml and methanol extract are 86.99 μg/ml in Table 1. Further experiments have been carried out with the KB cell line to study the anticancer activity of tulsi extract (1000, 500, 100, 30, 10 and 1 μg/ml). Both the extracts exhibited promising activity with IC50 values of less than 100 μg/ml in Table 2.
Morphological Observation
The light microscopic observation of extracts treated with KB oral cancer cell line after 48 hours of exposure showed typical morphological features of apoptosis as aqueous concentrations increased (100,300 and 10 μg/ml). The cell death inducing ability of both extracts is determined by visual observation under inverted microscope. This is indicated by formation of fragmented apoptotic bodies which are highly condensed. In this study that the number of condensed nuclei in hexane extract of Tulsi leaves was nearly higher than that of methanol extract in KB oral cancer cells. The untreated control cell lines showed no condensed or fragmented nuclei (Figure 3 and 4).
Phytochemical analysis
We have concluded the presence of Flavonoids, Saponins, Phenolic compounds and Steroids in Table 3.
Thin layer chromatography
Thin layer chromatography was performed for Tulsi Hexane extract to separate the phenolic compounds from the crude extract. Toluene: ethyl acetate: formic acid (36:12:5) [40] is used in order to separate phenolic compounds and the image represented in Figure 5. The Rf value of separated bands after exposure to iodine of yellow range is 0.97 and green shade 0.84 viewed in all three light rays.
p53 expression levels by Western blotting
The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading were upregulated and downregulated, respectively, in hexane tulsi extract treated cancer cells in Figure 6. A representative blot was shown from three independent experiments. The increase in expression of p53 can have an effect on the apoptosis of KB cell line (Oral cancer). From the result we found out that there is an increase in the expression of p53 at 100ug/ml and 300 ug/ml concentration of Tulsi Hexane extract treated cells.
|
Table 1. Cytotoxicity effect of Ocimum sanctum (Tulsi) Hexane and methanol extract in L 132 cell line. |
|||
|
Concentration (μg/ml)-L132-Tulsi Hexane extract |
% Live cell |
Concentration (μg/ml)-L132-Tulsi Methanol extract |
% Live cell |
|
1000 |
67.75 |
1000 |
59.18 |
|
300 |
71.14 |
300 |
67.05 |
|
100 |
71.99 |
100 |
77.78 |
|
30 |
82.02 |
30 |
85.57 |
|
10 |
89.27 |
10 |
91.05 |
|
1 |
94.68 |
1 |
95.45 |
|
IC 50 |
431.39 |
IC 50 |
86.99 |
|
Table 2. Anticancer activity of Ocimum sanctum (Tulsi) hexane and methanol extract in KB cell line. |
|||
|
Concentration (μg/ml)-L132-Tulsi Hexane extract |
% Live cell |
Concentration (μg/ml)-L132-Tulsi Methanol extract |
% Live cell |
|
1000 |
67.75 |
1000 |
59.18 |
|
300 |
71.14 |
300 |
67.05 |
|
100 |
71.99 |
100 |
77.78 |
|
30 |
82.02 |
30 |
85.57 |
|
10 |
89.27 |
10 |
91.05 |
|
1 |
94.68 |
1 |
95.45 |
|
IC 50 |
431.39 |
IC 50 |
86.99 |
|
Table 3. Phyto molecules present in Ocimum sanctum (Tulsi) Hexane extract. |
|
|
Phytochemical components |
Present/Absent |
|
Flavonoids |
+ |
|
Alkaloids |
- |
|
Saponins |
+ |
|
Tannins |
- |
|
Phenolic compounds |
+ |
|
Terpenoids |
- |
|
Steroids |
+ |
|
Glycosides |
- |
 Figure 1. Crude extracts of five different plant leaves (Azadirachta indica (Neem), Mangifera indica (Mango), Ocimum sanctum (Tulsi), Carica papaya (Papaya), and Senna auriculate (Avaram).
Figure 1. Crude extracts of five different plant leaves (Azadirachta indica (Neem), Mangifera indica (Mango), Ocimum sanctum (Tulsi), Carica papaya (Papaya), and Senna auriculate (Avaram).
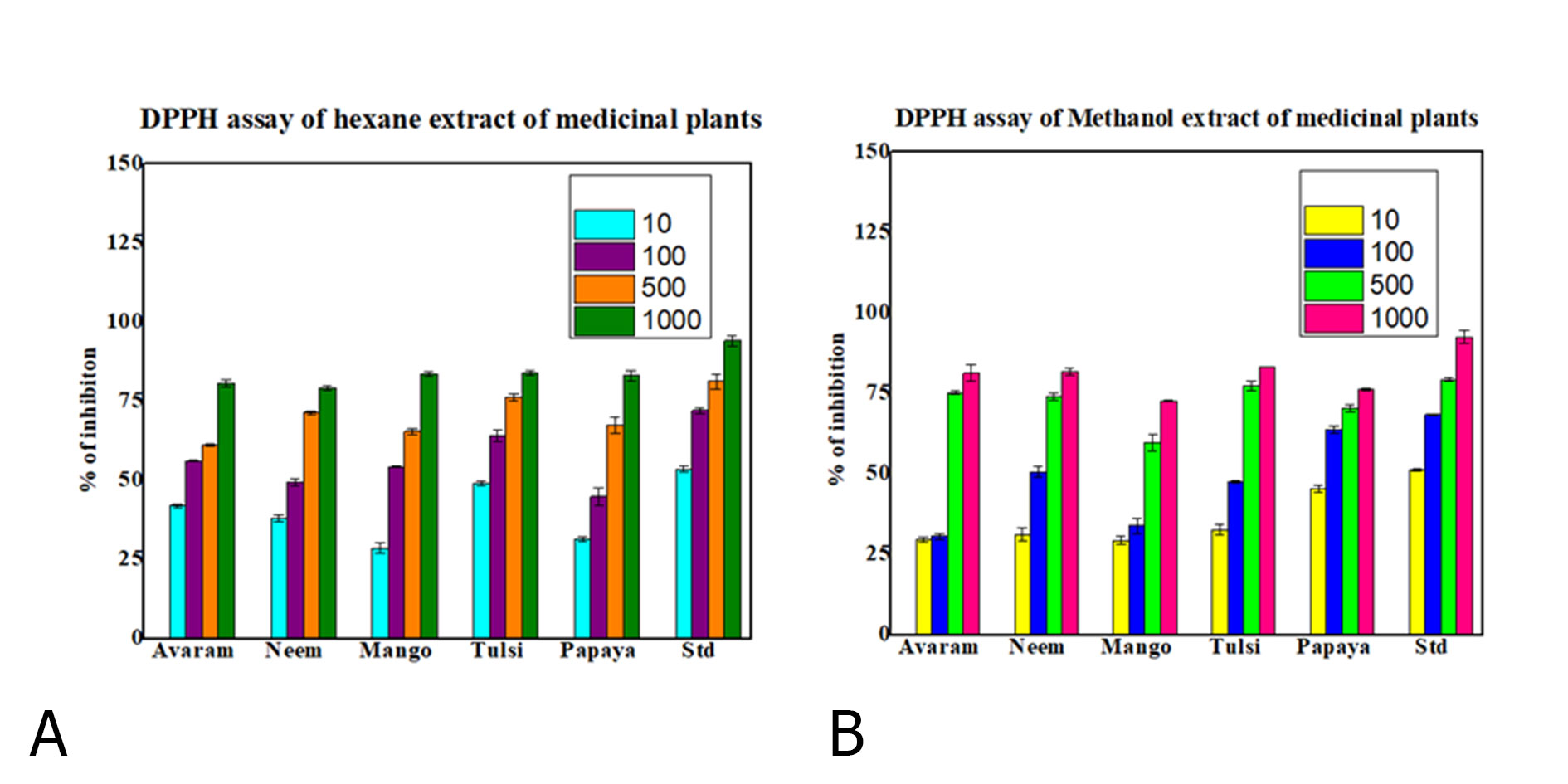 Figure 2. Percentage of inhibition of selected medicinal plant extract Azadirachta indica (Neem), Mangifera indica (Mango), Ocimum sanctum (Tulsi), Carica papaya (Papaya), and Senna auriculate (Avaram) in hexane (A)) and methanol (B) solvent on DPPH assay.
Figure 2. Percentage of inhibition of selected medicinal plant extract Azadirachta indica (Neem), Mangifera indica (Mango), Ocimum sanctum (Tulsi), Carica papaya (Papaya), and Senna auriculate (Avaram) in hexane (A)) and methanol (B) solvent on DPPH assay.
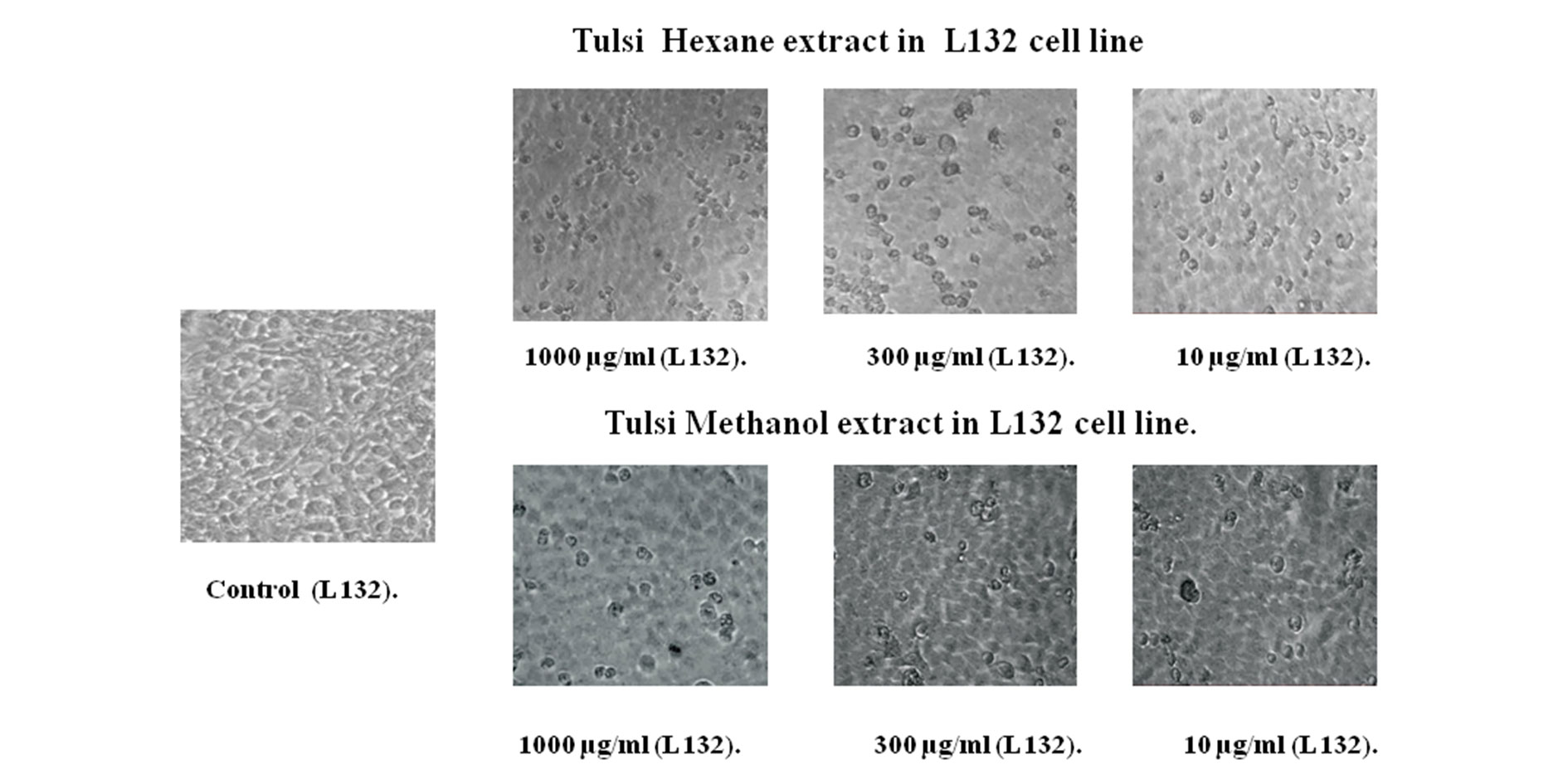 Figure 3. Ocimum sanctum (Tulsi) hexane and methanol extract treated in LI32 cell line using cell viability assay.
Figure 3. Ocimum sanctum (Tulsi) hexane and methanol extract treated in LI32 cell line using cell viability assay.
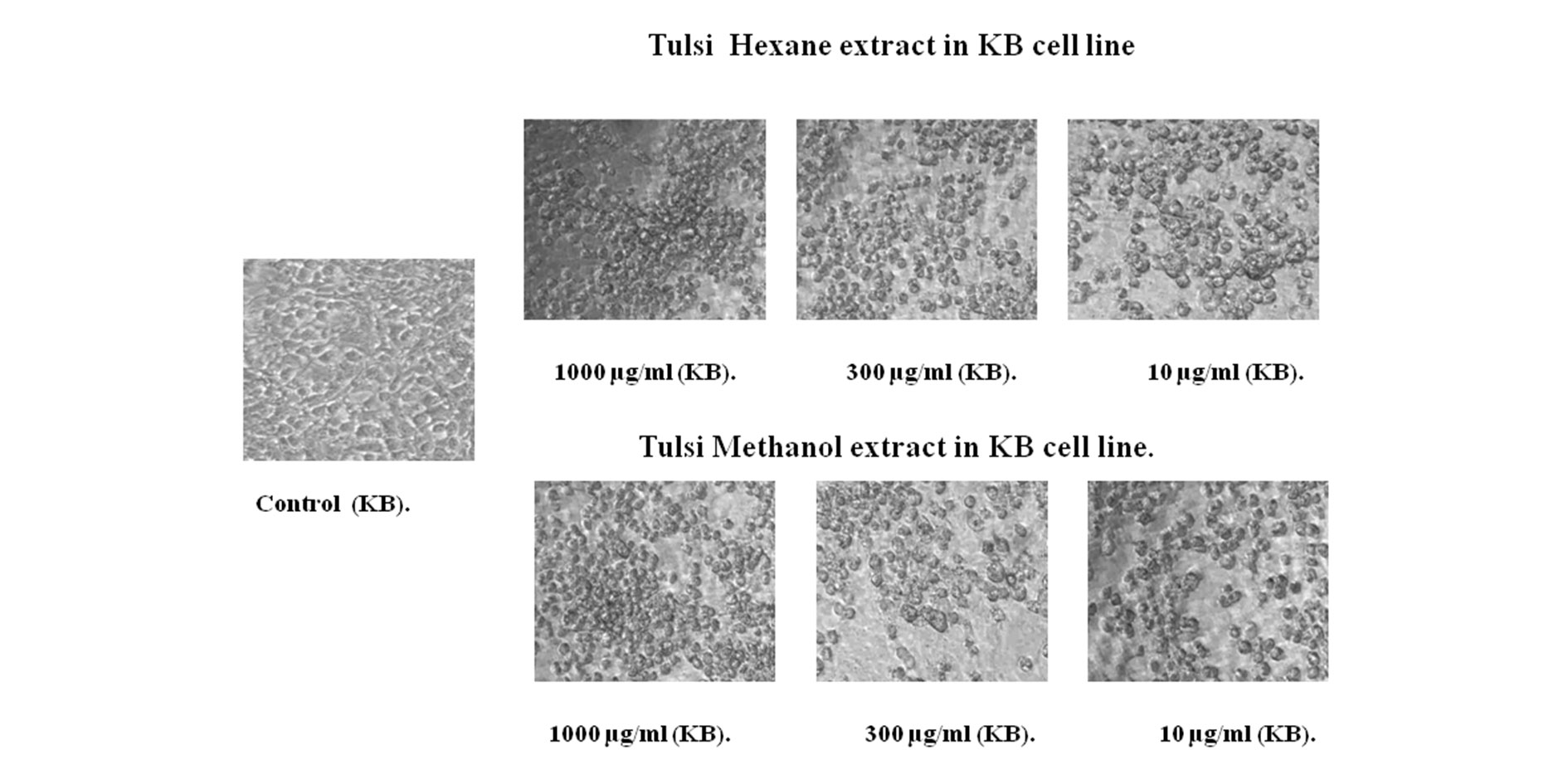 Figure 4. Ocimum sanctum (Tulsi) hexane and methanol extract treated in KB cell line MTT assay.
Figure 4. Ocimum sanctum (Tulsi) hexane and methanol extract treated in KB cell line MTT assay.
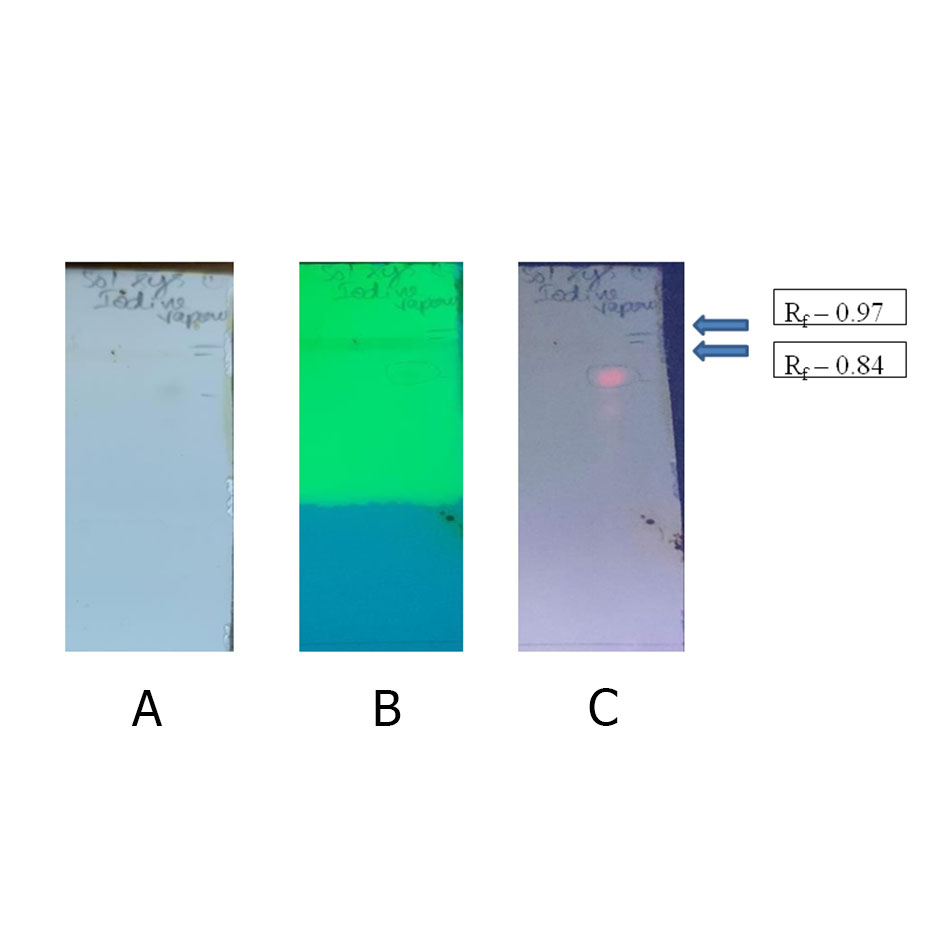 Figure 5. TLC after exposure to iodine vapor at (a) visible light, (b) UV-254 nm and (c) UV-366 nm and Rf factor is the distance travelled by the compound divided by the distance travelled by the solvent were mentioned in the right corner of the TLC sheet.
Figure 5. TLC after exposure to iodine vapor at (a) visible light, (b) UV-254 nm and (c) UV-366 nm and Rf factor is the distance travelled by the compound divided by the distance travelled by the solvent were mentioned in the right corner of the TLC sheet.
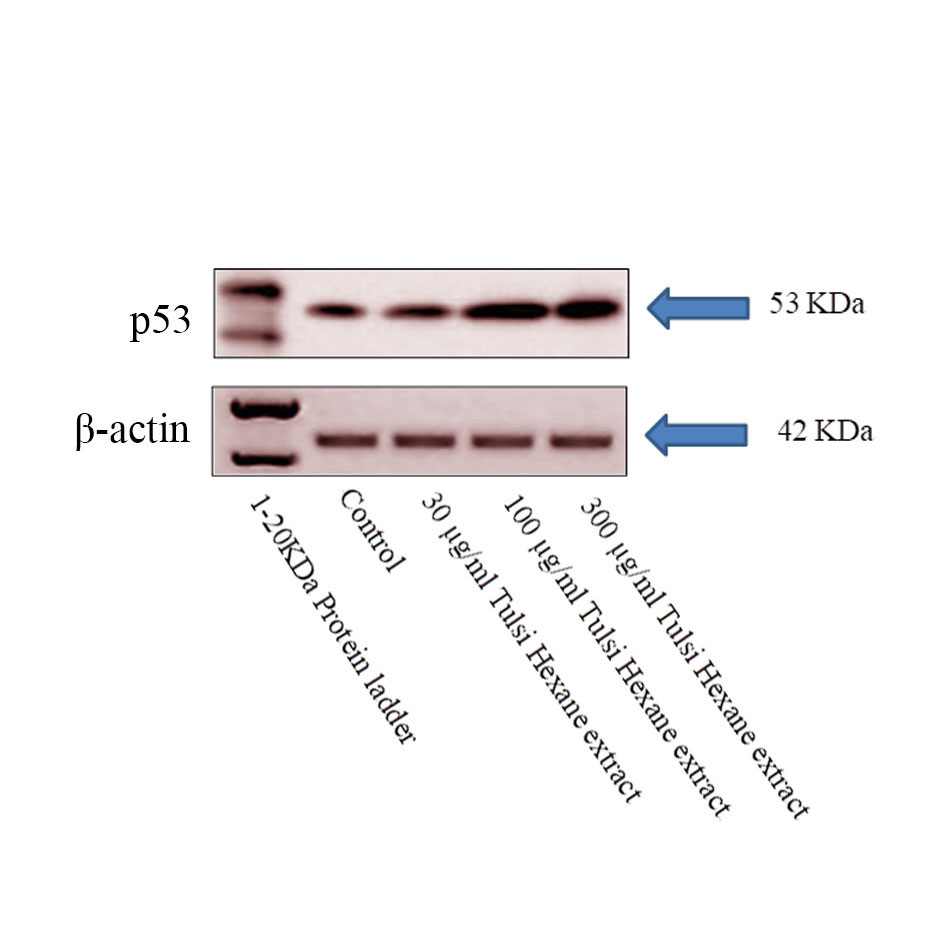 Figure 6. p53 expression in western blot, Apoptosis in oral cancer cells induced by the Hexane extract of Tulsi. Lysates of the KB cells treated with extract (30,100 and 300 μg/mL) for 24 and 48 h were subjected to an immunoblot analysis with antibodies against p53 tumor protein. Beta actin served as the loading control.
Figure 6. p53 expression in western blot, Apoptosis in oral cancer cells induced by the Hexane extract of Tulsi. Lysates of the KB cells treated with extract (30,100 and 300 μg/mL) for 24 and 48 h were subjected to an immunoblot analysis with antibodies against p53 tumor protein. Beta actin served as the loading control.
To gain a clearer understanding of the anti-oral cancer mechanism of hexane extract of Tulsi treated cancer cells, we investigated the effects of the tulsi extract on the expression levels and activities of intracellular signaling molecules. Cells were incubated with the indicated concentrations of hexane extract for 24 h and the whole cell lysates were analysed by western blotting using antibodies as indicated. In our study, we treated KB cells were seen under the microscope disclose the cell shrinkage and apoptotic body formation denote the characteristic of apoptosis. Whereas in the control group, the cells were regular in morphology and grew fully in patches and were confluent, rarely sloughing off. The same result was reported by Elina et al [49] in the aqueous extract of tulsi leaves. The apoptotic regulation is mainly depends upon the interbalance between Bcl-2 and Bax /p53 activity and also the other genes of their family for example Bad, Bcl-XL, Bcl-Xs and BAG1. These results suggested that the mitochondrial pathway may be involved in tulsi hexane extract induced KB cancer cell death. Mitochondria are Principal signaling centers throughout apoptosis and the loss of mitochondrial probity can be induced or hindered by numerous controllers of apoptosis where the P53 played vital role [50].
This article does not contain any studies with human participants or animals performed by any of the authors.
Author contributions
VRN carried out the entire lab work assays for the manuscript; JJ prepared draft document and invigilation for the manuscript; MSA carried out the extract preparation and antioxidant assay for the manuscript and VRK carried out correction and invigilation for the manuscript.
Competing interests
All authors declare no competing interests.
Acknowledgments
The authors are grateful to the management of Sathyabama Institute of Science and Technology (deemed University) Chennai for the providing the infrastructure facility.
- Fidler MM, Bray F, Soerjomataram I: The global cancer burden and human development: A review. Scand J Public Health 2018, 46(1): 27-36.
- WHO 2018. Cancer. In. http://www.who.int/mediacentre/factsheets/fs297/en/.
- Salahshourifar I, Vincent-Chong VK, Kallarakkal TG, Zain RB: Genomic DNA copy number alterations from precursor oral lesions to oral squamous cell carcinoma. Oral Oncol 2014, 50(5): 404-412.
- Tandon P, Dadhich A, Saluja H, Bawane S, Sachdeva S: The prevalence of squamous cell carcinoma in different sites of oral cavity at our Rural Health Care Centre in Loni, Maharashtra - a retrospective 10-year study. Contemp Oncol (Pozn) 2017, 21(2): 178-183.
- Sankaranarayanan R, Ramadas K, Amarasinghe H, Subramanian S, Johnson N: Oral cancer: prevention, early detection, and treatment. Cancer: disease control priorities, third edition (Volume 3) 2015; 85-99.
- Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Badwe R, Kumar R, Roy S, Suraweera W, Bray F, et al: Cancer mortality in India: a nationally representative survey. Lancet 2012, 379(9828): 1807-1816.
- Karbownik A, Szałek E, Urjasz H, Głęboka A, Mierzwa E, Grześkowiak E: The physical and chemical stability of cisplatin (Teva) in concentrate and diluted in sodium chloride 0.9%. Contemp Oncol (Pozn) 2012, 16(5): 435-439.
- Hampel S, Kunze D, Haase D, Krämer K, Rauschenbach M, Ritschel M, Leonhardt A, Thomas J, Oswald S, Hoffmann V, et al: Carbon nanotubes filled with a chemotherapeutic agent: a nanocarrier mediates inhibition of tumor cell growth. Nanomedicine (Lond) 2008, 3(2): 175-182.
- Šuleková M, Váhovská L, Hudák A, Žid L, Zeleňák V: A Study of 5-Fluorouracil Desorption from Mesoporous Silica by RP-UHPLC. Molecules 2019, 24(7): 1317.
- Katsuki MO, Masao.: Improvement Of The Water-Solubility Of Paclitaxel With An Amorphous Solid Dispersing Technique Using Polyvinylpyrrolidone As Hydrophilic Carrier10. 2019 Epub ahead of print.
- Alken S, Kelly CM: Benefit risk assessment and update on the use of docetaxel in the management of breast cancer. Cancer Manag Res 2013, 5: 357-365.
- Karimi M: Hydroxyurea in the management of thalassemia intermedia. Hemoglobin 2009, 33 Suppl 1: S177-182.
- Cirillo G, Vittorio O, Kunhardt D, Valli E, Voli F, Farfalla A, Curcio M, Spizzirri UG, Hampel S: Combining Carbon Nanotubes and Chitosan for the Vectorization of Methotrexate to Lung Cancer Cells. Materials (Basel) 2019, 12(18): 2889.
- He Y, Lan Y, Liu Y, Yu H, Han Z, Li X, Zhang L: Pingyangmycin and Bleomycin Share the Same Cytotoxicity Pathway. Molecules 2016, 21(7): 862.
- Meloun M, Ferenčíková Z, Vrána A: Determination of the thermodynamic dissociation constant of capecitabine using spectrophotometric and potentiometric titration data. J Chem Thermodyn 2011, 43(6): 930-937.
- Islam M, Datta J, Lang JC, Teknos TN: Down regulation of RhoC by microRNA-138 results in de-activation of FAK, Src and Erk1/2 signaling pathway in head and neck squamous cell carcinoma. Oral Oncol 2014, 50(5): 448-456.
- Amit Kumar AK, Anu Rahal AR, Sandip Chakraborty SC, Ruchi Tiwari RT, Latheef SK, Kuldeep Dhama KD: Ocimum sanctum (Tulsi): a miracle herb and boon to medical science-a review. Int J Agron Plant Prod 2013, 4(7): 1580-1589.
- Sivaraj R, Rahman PK, Rajiv P, Narendhran S, Venckatesh R: Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim Acta A Mol Biomol Spectrosc 2014, 129: 255-258.
- Ochwang'i DO, Kimwele CN, Oduma JA, Gathumbi PK, Mbaria JM, Kiama SG: Medicinal plants used in treatment and management of cancer in Kakamega County, Kenya. J Ethnopharmacol 2014, 151(3): 1040-1055.
- Kamatou GP, Van Zyl R, Davids H, Van Heerden F, Lourens A, Viljoen AM: Antimalarial and anticancer activities of selected South African Salvia species and isolated compounds from S. radula. S Afr J Bot 2008, 74(2): 238-243.
- Greenwell M, Rahman PK: Medicinal Plants: Their Use in Anticancer Treatment. Int J Pharm Sci Res 2015, 6(10): 4103-4112.
- Shirai Y, Fujita Y, Hashimoto K: Effects of the antioxidant sulforaphane on hyperlocomotion and prepulse inhibition deficits in mice after phencyclidine administration. Clin Psychopharmacol Neurosci 2012, 10(2): 94-98.
- Jagu E: Design, synthesis and biological evaluation of new polyamine derivatives as antikinetoplastid agents. Université Paris Saclay (COmUE); 2016.
- Arora R, Malhotra P, Mathur AK, Mathur A, Govil C, Ahuja P: Anticancer alkaloids of Catharanthus roseus: transition from traditional to modern medicine. Herbal Medicine: A Cancer Chemopreventive and Therapeutic Perspective Jaypee Brothers Medical Publishers Pvt Ltd, New Delhi, India 2010Epub ahead of print.: 292-310.
- Peterson C, Coats J: Insect repellents-past, present and future. Pesticide Outlook 2001, 12(4): 154-158.
- Dettlaff K, Stawny M, Ogrodowczyk M, Jelińska A, Bednarski W, Wątróbska-Świetlikowska D, Keck RW, Khan OA, Mostafa IH, Jankun J: Formulation and characterization of EGCG for the treatment of superficial bladder cancer. Int J Mol Med 2017, 40(2): 329-336.
- Zweifel M, Rustin G: The clinical development of tubulin binding vascular disrupting agents. Vascular disruptive agents for the treatment of cancer 2010Epub ahead of print.: 183-216.
- Zhai S, Senderowicz AM, Sausville EA, Figg WD: Flavopiridol, a novel cyclin-dependent kinase inhibitor, in clinical development. Ann Pharmacother 2002, 36(5): 905-911.
- Naik PK, Santoshi S, Joshi HC: Noscapinoids with anti-cancer activity against human acute lymphoblastic leukemia cells (CEM): a three dimensional chemical space pharmacophore modeling and electronic feature analysis. J Mol Model 2012, 18(1): 307-318.
- Shah U, Shah R, Acharya S, Acharya N: Novel anticancer agents from plant sources. Chin J Nat Med 2013, 11(1): 16-23.
- Amin A, Gali-Muhtasib H, Ocker M, Schneider-Stock R: Overview of major classes of plant-derived anticancer drugs. Int J Biomed Sci 2009, 5(1): 1-11.
- Brand-Williams W, Cuvelier M-E, Berset C: Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 1995, 28(1): 25-30.
- Miladi S DM: In vitro antioxidant activities of Aloe vera leaf skin extracts. J Soc Chim Tunisie. 2008;10(10):101-109. J Soc Chim Tunisie 2008, 10(10): 101-109.
- Garcia EJ, Oldoni TL, Alencar SM, Reis A, Loguercio AD, Grande RH: Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz Dent J 2012, 23(1): 22-27.
- Gopakumar D, Nair SS: Aloe vera is non-toxic to cells: A microculture tetrazolium technique colorimetric assay study. J Indian Acad Oral Med Radiol 2014, 26(4): 364-368.
- Ali Hassan SH, Abu Bakar MF: Antioxidative and anticholinesterase activity of Cyphomandra betacea fruit. Sci World J 2013, 2013: 278071.
- Trease G, Evans W: Phytochemical screening: Textbook of pharmacognosy. London: Bailiere Tindal Limited 1989 Epub ahead of print.: 191.
- Singh D, Singh P, Gupta A, Solanki S, Sharma E, Nema R: Qualitative estimation of the presence of bioactive compound in Centella asiatica: an important medicinal plant. Int J Life Sci Med Sci 2012, 2(1): 5-7.
- Wadood A, Ghufran M, Jamal SB, Naeem M, Khan A, Ghaffar R: Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem anal biochem 2013, 2(4): 1-4.
- Medić-Šarić M, Jasprica I, Smolčić-Bubalo A, Mornar A: Optimization of chromatographic conditions in thin layer chromatography of flavonoids and phenolic acids. Croat Chem Acta 2004, 77(1-2): 361-366.
- DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, Jemal A: Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin 2016, 66(4): 290-308.
- Hu W, Kavanagh JJ: Anticancer therapy targeting the apoptotic pathway. Lancet Oncol 2003, 4(12): 721-729.
- Tseng PY, Lu WC, Hsieh MJ, Chien SY, Chen MK: Tanshinone IIA induces apoptosis in human oral cancer KB cells through a mitochondria-dependent pathway. Biomed Res Int 2014, 2014: 540516.
- Balaji R, Prakash G, Suganya P, Aravinthan K: Antioxidant activity of methanol extract of Ocimum tenuiflorum (dried leaf and stem). Int J Pharm Sci Rev Res 2011, 3: 20-27.
- Green DR, Reed JC: Mitochondria and apoptosis. Science 1998, 281(5381): 1309-1312.
- Hu W, Kavanagh JJ: Anticancer therapy targeting the apoptotic pathway. Lancet Oncol 2003, 4(12): 721-729.
- Joseph B, Nair VM: Ocimum sanctum Linn.(Holy basil): pharmacology behind its anti-cancerous effect. Int J Pharm Bio Sci 2013, 4(2): 556-575.
- Mishra N, Tapas Khuntia TK: The ethno pharmacological activity of Tulsi (Ocimum sanctum): a review of recent research. Int J Drug Formul Res 2014, 5(2): 24-52.
- Mitra E, Ghosh D, Ghosh AK, Basu A, Chattopadhyay A, Pattari SK, Datta S, Bandyopadhyay D: Aqueous Tulsi leaf (Ocimum sanctum) extract possesses antioxidant properties and protects against cadmium-induced oxidative stress in rat heart. Int J Pharm Pharm Sci 2014, 6(1): 500-513.
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ: Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 1995, 80(2): 285-291.
Asia-Pacific Journal of Oncology
print ISSN: 2708-7980, online ISSN: 2708-7999
 Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript