Review | Open Access
Deubiquitinases as novel therapeutic targets in colorectal cancer
Noor Al Shukri1, Razik Bin Abdul Momin1
1Qatar Biomedical Research Institute, Histopathology Unit, Hamad Bin Khalifa University, Al Rayyan District, P.O Box 34110 Doha, Qatar.
Correspondence: Razik Bin Abdul Momin (Qatar Biomedical Research Institute, Histopathology Unit, Hamad Bin Khalifa University, Al Rayyan District, P.O Box 34110 Doha, Qatar; E-mail: raabdulmuumin@hbku.edu.qa).
Asia-Pacific Journal of Oncology 2025, 6: 27-36. https://doi.org/10.32948/ajo.2025.02.19
Received: 21 Dec 2024 | Accepted: 15 Mar 2025 | Published online: 17 Mar 2025
Key words deubiquitinase, colorectal cancer, protein stability, tumor microenvironment, chemotherapy resistance, targeted therapy
Modifications of proteins after translation are closely related to tumorigenesis and progression, therefore post-translational modification (PTM) of proteins has attracted increasing attention in recent years. In addition, protein stability and function are regulated by changes in ubiquitination and deubiquitination, as well as ubiquitin addition and removal are critical for biological processes such as cell cycle, apoptosis, autophagy and immune responses [5, 6]. Deubiquitinating enzymes (DUBs), have been discovered to be essential for maintaining protein structure and regulating diverse signaling pathways [7].
Deubiquitination alters ubiquitination modifications and removes ubiquitin molecules from target proteins via DUBs, thereby controlling protein degradation and subcellular localization. Over the past few years, studies have shown significant effects of ubiquitination and deubiquitination on the post-translational functions of proteins, especially some proteins are crucial for signal transduction, regulation of gene expression and cellular homeostasis, which has further stimulated the research interest [8, 9]. Ubiquitination modification is a process in which a number of enzymes (E1, E2, and E3) attach ubiquitin molecules to target proteins [10]. In addition to being essential for regular cellular physiological functions, DUBs are also intricately associated with the initiation and development of a number of illnesses, particularly cancer [11].
Research has shown a significant correlation between DUBs and the development and incidence of a number of malignancies, such as CRC and other tumors of the digestive system [12]. In CRC, DUBs promote tumor invasion and metastasis by maintaining the equilibrium of cancer cell proliferation, apoptosis, autophagy, and immune microenvironment. In addition, some DUBs are also associated with chemotherapy resistance, such as by maintaining the stability of drug target proteins or regulating apoptosis-related pathways, mediating a decrease in sensitivity of CRC cells to chemotherapy drugs [13, 14]. Therefore, DUBs are not only an important pathogenic factor for CRC, but also have the potential to become a novel therapeutic target. As the functions of DUBs are gradually revealed, their value as potential therapeutic targets for CRC is also receiving increasing attention. Low-molecular-weight inhibitors for DUBs, such as USP7, USP14, and WP1130 inhibitors, have made considerable headway in recent years, and have demonstrated strong anti-tumor effects in a variety of cancer models [15]. However, these inhibitors still face challenges in terms of selectivity, toxicity, and pharmacokinetic properties, limiting their widespread clinical applications.
Although many studies have demonstrated the role of DUBs in CRC, little is known about their molecular regulatory networks and mode of action. In addition, some side effects triggered in response to DUB treatment need to be urgently addressed. Here, we summarize the progress of DUBs in CRC research in recent years and the roles they play in tumorigenesis, drug resistance and immunomodulation, and discuss their potential applications in the treatment of CRC.
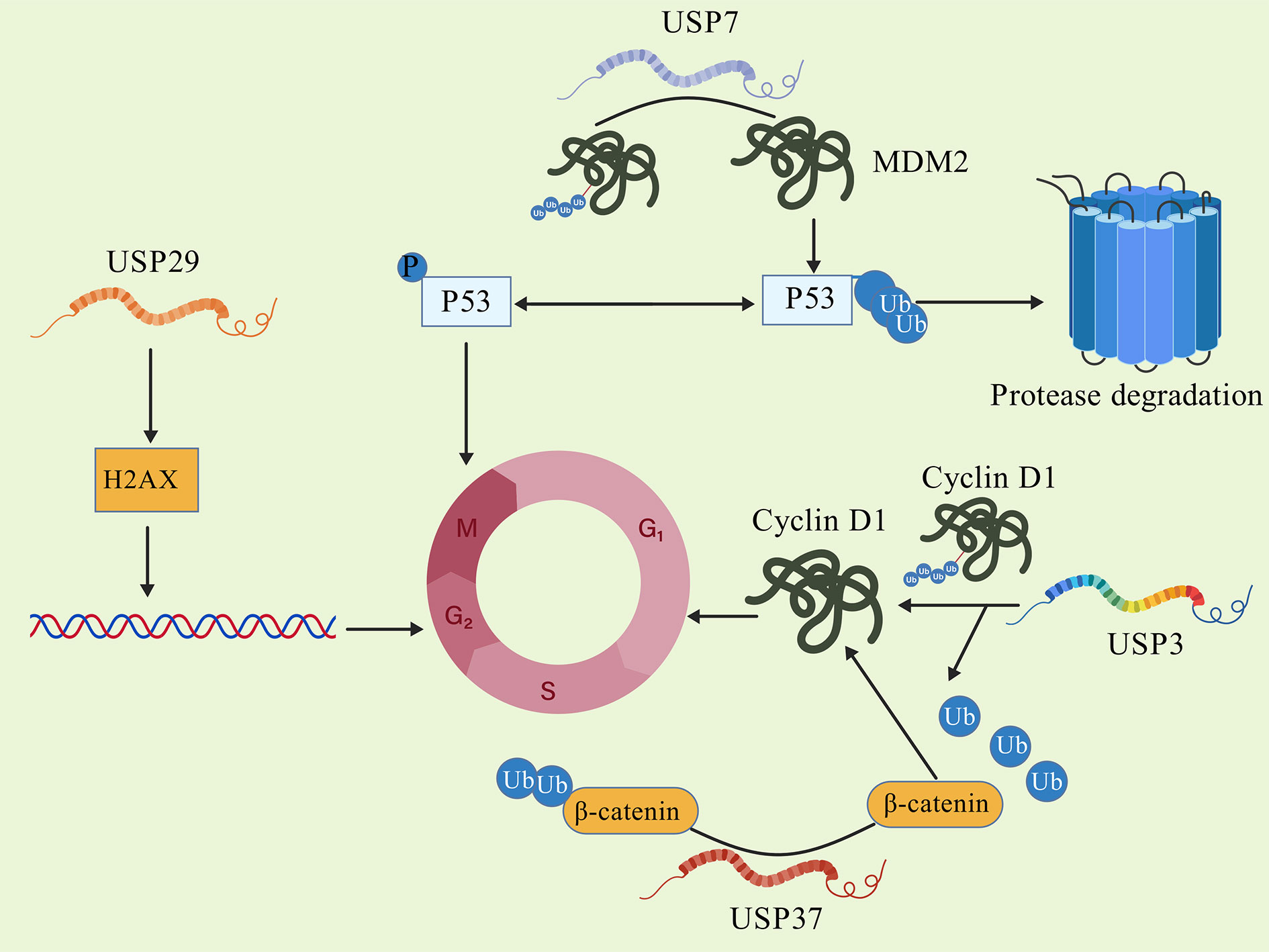 Figure 1. DUBs promote cell cycle progression.
Figure 1. DUBs promote cell cycle progression.
(1) Ubiquitin specific protease family (USPs)
USPs constitute the largest subgroup of DUBs with diverse members involved in controlling the regulation of signaling pathways as well as maintaining protein homeostasis. It has been shown that USP7 is an important deubiquitinating enzyme in CRC, and its high expression is strongly associated with the unfavorable prognosis of CRC patients. In addition to USP7, the roles of USP10 and USP22 in CRC have also been thoroughly investigated. In addition, it regulates the expression of p53 or MDM2, thereby controlling cell division and death. According to Al Eidan, USP7 functions in CRC to control intercellular adhesion through AJUBA. The results of the study showed that when USP7 was knocked down, CRC cell survival and intercellular adhesion were significantly reduced [19]. Furthermore, according to Zhang et al, the results showed that USP7 ubiquitinates and modulates MyD88 expression, which improves the immune response. In addition, this study showed that USP7 promotes CRC tumor cell invasion and metastasis through the activation of the Wnt/β-catenin pathway via deubiquitinating β-catenin [20]. To investigate the relationship between USP7 and YY1 in CRC, Shao et al. found that USP7 accelerates the development of CRC by activating the Wnt/β-catenin pathway, thus revealing their intricate modes of action in CRC [21].
USP10 promotes CRC tumor growth through improving the polarization of tumor-associated macrophages. According to Cao and Peng's research, USP10 and OTUB2 deubiquitinate NLRP7 and PKM2, speeding up the progression of CRC. Furthermore, the study focused at the potential prognostic role of these genes in the development and spread of CRC [22]. According to Kubaichuk's findings, patients with CRC who had high USP10 expression experienced significantly poorer overall and recurrence-free survival rates, implying that USP10 may play a role in tumor progression. Further research suggests that knocking out USP10 can alleviate the invasive phenotype of HCT116 colon cancer cells [23]. USP10 is also associated with p53, and studies have shown that it has anti-cancer properties in some cases by stabilizing wild-type p53, but it is more likely to have a pro-cancer effects in CRC [24].
Through controlling the stability of cell cycle proteins and c-Myc, USP22, a DUB that is abundantly expressed in CRC, stimulates tumor cell proliferation and advances the cell cycle. Furthermore, Liu Y's research has shown that low prognosis in advanced AJCC staging was substantially linked to CRCs, with elevated mRNA levels of USP22, BMI-1, c-Myc, and cyclin D2 [25]. USP22 also promotes the metastasis of CRC cells by regulating the expression of EMT (epithelial mesenchymal transition) related genes. Li et al. reported that overexpression of USP22 in CRC is associated with liver metastasis, and found that USP22 activates EMT via upregulating AP4 expression, thereby, enhancing the migration and invasion ability of CRC cells. They also pointed out that USP22 activates AP4 transcription by directly binding to its promoter region [26].
(2) Ubiquitin C-terminal hydrolase family (UCHs)
Members of the UCHs family include UCH-L1 and UCH-L3, which play important roles in tumor-related signaling pathway. Lee KC et al. analyzed the levels of expression of CHGA and UCH-L1 in CRC tissues using iTRAQ technology and found that the high expression of these two proteins is closely related to lymph node metastasis [27]. Furthermore, Ma Y's research indicated that UCH-L1 represents a functional protein that might be crucial for cell migration, in addition to being a biomarker for lymph node metastasis in CRC [28]. UCH-L1 exhibits a pro-cancer effect in CRC by stabilizing inflammatory factors in the tumor microenvironment (TME) or regulating the migration ability of cancer cells [29]. There are few reports on the function of UCH-UCH-L3 in CRC. According to Nam MJ's research, UCH-L3 antibodies were found in the serum of 19 out of 43 individuals with colon cancer. Nonetheless, they may have an impact on tumor cell survival by controlling the stability of proteins linked to apoptosis [30].
(3) The deubiquitinase family (OTUs) and other families containing OTU domains
OTUs family fine-tunes diverse signaling pathways, and its members such as OTUB1 and OTUB2 have received widespread attention in the study of CRC. Wu's research found that OTUB1 stabilizes the expression of MSH2 by preventing its ubiquitin-mediated degradation, leading to affect the DNA mismatch repair process, and contributing to CRC cells' resistance to treatment [31]. In addition, Zhu D's research has shown that OTUB1 stimulates the proliferation of cancer cells and inhibits apoptosis by deubiquitinating specific substrates, suggesting its important role in tumor progression [32]. Wang D found that the expression of circSEC24B is elevated in CRC cell lines and tissues, and promotes CRC cell autophagy and proliferation. By controlling the protein stability of SRPX2, circSEC24B mechanistically stimulates the growth of CRC cells. As a scaffold, circSEC24B specifically facilitates OTUB1's binding to SRPX2, increasing OTUB1's protein stability. In summary, this study suggests that circSEC24B stimulates autophagy and triggers chemotherapy resistance in CRC by promoting OTUB1 mediated deacetylation of SRPX2 [33]. Zhu's research found that OTUB2 is significantly expressed in CRC tissues and is linked to a poor prognosis in patients. Functional experiments have shown that knocking down OTUB2 can weaken the stemness of CRC cells, enhance their sensitivity to oxaliplatin, inhibit EMT processes, and thus, reduce tumor metastatic ability. Mechanistic studies have shown that OTUB2, as a deubiquitinase, directly interacts with transcription factor SP1, inhibiting its K48 ubiquitination and stabilizing SP1 protein. SP1 further acts as a transcription regulator for GINS1, promoting its transcriptional activity and ultimately regulating the stemness, and antineoplastic drug refractoriness coupled with EMT dynamics in CRC [34].
The MJD family is mainly involved in the clearance of protein aggregates and degraded proteins. In CRC, there is limited research on the MJD family, but some preliminary studies suggest that it may function by regulating stress-related proteins [35]. For example, Chen, Y's research found that glucosidase I (GCS1) recruits the deubiquitinase USP10 to deubiquitinate GRP78, promoting its degradation and alleviating endoplasmic reticulum stress, thereby, promoting the progression of CRC [35]. The JAMMs family plays a role in DNA damage repair and signal transduction. For example, BRCC36 plays a key role in controlling cellular DNA damage response [36], though its specific mechanism in CRC is not yet clear. MINDYs is a newly discovered family of deubiquitinases that primarily recognize specific chain types of ubiquitin chains. In CRC, their potential functions and mechanisms still need further research.
The steady-state control of proteins linked to the onset and progression of CRC is directly affected by the ubiquitin proteasome system (UPs). It is made up of DUBs, the 26S proteasome, ubiquitin conjugating enzymes (E2), ubiquitin ligase enzymes (E3), ubiquitin activating enzyme (El) and the small molecule ubiquitin (Ub), which can control protein degradation and are key regulatory systems for protein function and stability [41]. Ub can covalently connect to the lysine residue's amino group of the receptor protein through its C-terminal glycine carboxyl group, a process involving enzymes E1, E2, and E3. E1 is activated by adenosylation of ATP at the C-terminus of protein Ub. The activated protein binds to E2 through a thioester bond, and finally E3 mediates the formation of an heteropeptide bond between the carboxyl terminus of protein Ub and the lysine of the substrate protein, completing the ubiquitination modification of the protein [42]. Through peptide/heteropeptide interactions, Ub's seven lysine residues (K6, K11, K27, K29, K33, K48, and K63) and N-terminal methionine residue (M1) can all form single, mixed, linear, or branching ubiquitin chains [43]. For instance, K63 connected ubiquitin chains primarily take part in processes like signal transduction and DNA repair, whereas K48 linked ubiquitin chains usually mark proteins for proteasomal breakdown [44]. In addition, the ubiquitin chain connected to K33 has been found to play a critical role in intracellular material transport. In addition, the diversity of ubiquitin chains is also reflected in their topological structure, including homogeneous (only one type of connection) and heterogeneous (multiple types of connections) chains, as well as linear and branched structures. This structural diversity enables ubiquitination modification to finely regulate various biological processes within cells [45]. Various ubiquitin chain types dictate the distinct outcomes of substrate proteins. Polyubiquitin chains connected through K48 and K11 are generally linked to proteasomal breakdown, whereas other kinds of polyubiquitin chains are implicated in autophagy, DNA damage response, and signal transduction [46].
Protein ubiquitination is a highly dynamic process. In contrast to the E1-E2-E3 ubiquitin ligase system, DUB can remove ubiquitins or polyubiquitin chains from the target proteins, thereby counteracting the ubiquitination process, and reversing protein fate and related physiological processes [47, 48]. The altered expression and activity of DUBs are closely related to cell cycle and immune regulation disorders, contributing to the initiation and advancement of CRC. The known DUBs in humans can currently be divided into 8 families. Understanding how these DUBs mediate cellular life activities can offer novel approaches for treating diseases such as CRC.
Promoting cell cycle progression
The process of the cell cycle is a highly controlled process, divided into four stages: G1, S, G2, and M phases. The cell cycle is driven by regulatory mechanisms in the nucleus, which rely on the orderly activation of multiple cell cycle proteins (cyclins) and cyclin dependent kinases (CDKs) [51]. Cell cycle dysregulation is one of the main characteristics of cancers, including CRC. Abnormal activation of cell cycle-related proteins in cancer leads to uncontrolled cell proliferation, ultimately resulting in the development of tumors [52]. Various DUBS exhibit abnormal activity in CRC, promoting cell proliferation and inhibiting apoptosis by regulating the cell cycle progression, becoming an indispensable driving force for the occurrence and development of CRC (Figure 1).
USP1 is markedly increased in the tumors tissues of CRC patients, Xu X et al. found that knocking down USP1 increases the level of phosphorylated p53 in HCT116 cells, leading to increased expression of downstream target protein, cyclin dependent kinase inhibitor p21, ultimately resulting in cell cycle G/M arrest [53]. Montalto et al. found that cyclin D1 can regulate the progression from the G phase to the S phase of the cell cycle [54]. Notably, USP2 stabilizes cyclin D1 directly through deubiquitinase activity, and knocking down USP2 significantly reduces the half-life of cyclin D1 and inhibits cell proliferation in HCT116 cells [55]. Xu et al. found that USP5 is highly expressed in CRC where it directly deubiquitinates and upregulates the expression of Tu translation elongation factor (TUFM)., In turn, TUFM upregulates cyclin D1 and promotes cell proliferation. Knocking down USP5 can lead to HCT116 cell growth inhibition [56]. As a unique deubiquitinase targeting both p53 and Mdm2, the protease HAUSP plays a crucial role in the regulation of p53 and is an essential part of the p53/Mdm2 pathway. Another important regulator of the p53 pathway is USP7 which deubiquitinates and promotes the stability of the ubiquitin ligase MDM2. MDM2 raises p53 ubiquitination levels, causing its destruction and, resulting in cell cycle progression and aberrant tumor cell growth [57]. Dai X's study has shown that USP7 inhibitors increase the expression of programmed death ligand 1 (PD-L1) in tumors, reprogram tumor-associated macrophages, and effectively inhibit programmed death protein 1 (PD-1) to control immune response against tumors [58]. The Al Eidan study revealed the role of USP7 as a cell adhesion regulator in CRC, indicating that USP7 regulates cell adhesion through targeting AJUBA [19].
USP29 is overexpressed in tumor tissues of CRC patient. Chandrasekaran knocked out USP29 in HCT116 cells, leading to an increase in the amount of DNA damage marker, phosphorylated histone H2AX (yH2AX), triggering genomic integrity impairment via bicentenary lesions with mitotic phase arrest, thereby reducing cell proliferation ability [59]. In various cancers, cell cycle progression is accelerated, cell proliferation is enhanced, and the cell cycle transition from the G phase to the S phase is facilitated by dysregulation of CDK4 [60]. Tong et al. found that USP37 is upregulated in the tumor tissues of CRC patients. Knocking down USP37 in HCT116 and T84 cells results in a reduction of cyclin D1 levels. Furthermore, it was found that USP37 participates in regulating the cell cycle by stabilizing the level of B-junction protein and upregulating the expression of cyclin D1, thereby promoting cell proliferation and leading to CRC progression [61].
Impact on signal pathways
In recent years, the abnormal activation of DUBs in CRC and their impact on signaling pathways have become research hotspots. DUBs regulate protein stability and function through the removal of ubiquitin molecules from proteins, thereby affecting cellular processes including proliferation, apoptosis, and migration. In CRC, abnormal expression or activity of DUBs can lead to dysregulation of multiple signaling pathways, promoting the occurrence and development of tumors [62].
The occurrence of CRC is significantly influenced by Wnt/β-catenin signaling pathway. Zhu, W.'s research found that USP4 improves the stemness of CRC cells through activating the Wnt/β-catenin pathway and facilitating the nuclear localization of β-catenin by deubiquitination [34]. Abnormal Wnt signaling pathway is strongly associated with tumor development, and can be classified into classical pathway and non-classical pathway based on whether it depends on β-catenin or not. The classical pathway, also known as the Wnt/β-catenin pathway, involves the entry of β-catenin into the nucleus and the binding of transcription factor TCF/LEF to initiate downstream target gene transcription, participating in the regulation of cell proliferation. In addition, Sun H et al. found that USP11 stabilizes its expression by deubiquitinating protein phosphatase PPP1CA, thereby. activating the ERK/MAPK signaling pathway and promoting the growth and metastasis of CRC cells [63].
Non-classical pathways such as the Wnt/Ca²⁺ pathway mainly regulate cell polarity and migration. In CRC, various DUBs directly intervene or indirectly affect the stability of intracellular β-catenin and nuclear aggregation, causing aberrant Wnt/β-catenin signaling activation, which encourages the migration and proliferation of cancer cells [64] (Figure 2).
The impact of deubiquitinase on drug resistance
Chemotherapy resistance in CRC remains a major hurdle in clinical treatment, significantly reducing patient survival rates and treatment outcomes. In recent years, studies have found that DUBs, as important regulatory factors in the ubiquitin proteasome system, participate in the formation of chemotherapy resistance through various mechanisms and are essential for the survival and adaptability of cancer cells.
DUBs play a significant role in regulating cancer cells to evade chemotherapy induced cell death. Taking USP9X as an example, Schwickart M research found that it stabilizes the pro-survival protein Mcl-1 through ubiquitination, inhibits the apoptosis pathway caused by chemotherapy drugs, and enhances the chemotherapy tolerance of cancer cells. The stability of Mcl-1 is crucial for the survival of chemotherapy resistant cancer cells, which not only involves the direct effects of chemotherapy drugs, but is also closely related to the cancer cells' ability to adapt to endogenous stress [65]. Small molecule inhibitors targeting USP9X have shown significant anti-tumor effects in experimental models, indicating that targeting DUBs may become a new strategy to overcome drug resistance. Harris DR used homologous recombination technology to disrupt the USP9X gene and found that CRC cells lacking USP9X were more sensitive to the chemotherapy drug 5-fluorouracil (5-FU). Inhibiting USP9X may increase the effectiveness of chemotherapy, as it may contribute to CRC cells' resistance to it [66]. In addition, the Cao Y study found that tanshinone IIA inhibits the Akt/WEE1/CDK1 signaling pathway, leading to downregulation of survivin phosphorylation and disruption of the interaction between USP1 and survivin, thereby inhibiting tumor growth in CRC and overcoming chemotherapy resistance [67].
DUBs directly affect the accumulation and action of drugs in cells by regulating the stability of drug efflux related proteins, such as P-glycoprotein and chemotherapy drug targets. For example, USP14 has been found to regulate the expression level of P-glycoprotein through the ubiquitin proteasome pathway. Overexpression of USP14 can significantly increase the activity of P-glycoprotein, thereby expelling chemotherapy drugs to the extracellular space and reducing intracellular drug concentration [68]. In addition, some DUBs indirectly weaken the efficacy of drugs by regulating the ubiquitination state of drug targets, enhancing their stability.
De ubiquitination and TME
The dynamic characteristics of TME have a pivotal role in various stages of tumor occurrence and development, among which DUBs profoundly affect the structure and function of TME by regulating the ubiquitination status of signaling molecules and metabolic pathways. Lately, there has been an increasing number of research reports revealing the specific mechanisms and potential therapeutic targets of DUBs in TME.
Chuang et al. highlighted the involvement of T lymphocytes in the pathophysiology and the advancement of CRC, along with the makeup of the tumor immune microenvironment in CRC, and also discussed the current T-cell related immunotherapy methods, emphasizing the importance of TME in CRC treatment [69]. On the other hand, Guo Xw analyzed the gene expression data of CRC, constructed the TME score (TMEscore), and divided the samples into different TME patterns. The results indicated that TMEscore was strongly correlated with clinical characteristics, prognosis, immune score, gene mutations, and immune checkpoint inhibitor response in patients. This indicates that TME features can be used to predict the prognosis and immune therapy response in CRC patients [70]. Wu et al. comprehensively characterized the TME of CRC, including the functional status, immune and stromal characteristics, and alterations in metabolic reprogramming of tumor cells. As a result, the study successfully classified CRC into four subtypes based on 61 TME related features. Comprehensive analysis showed that these subtypes have significant differences in histopathology, molecular characteristics, treatment efficacy, and prognosis [71].
Chronic inflammation in TME is an important driving factor for tumor development. Li B's study found that USP10 stabilizes the protein level of NLRP7 by deubiquitinating it, encouraging the growth and spread of CRC cells. In addition, NLRP7 overexpression is linked to tumor-promoting M2 macrophage polarization, revealing the important role of the USP10-NLRP7 axis in the CRC TME [14]. Wang XM's research shows that USP25 is crucial to colitis and bacterial infections, and its upregulation can promote the development of CRC. Inhibition of USP25 can enhance immune response, promote bacterial clearance and inflammation resolution, and weaken Wnt and SOCS3-pSTAT3 signaling, thereby inhibiting the occurrence of colon tumors [72]. Zhou Y's research found that USP4 is upregulated in microsatellite stable CRC and negatively regulates immune response against tumors. USP4 inhibits the nuclear localization of IRF3, suppresses interferon response and antigen presentation, and weakens pattern recognition receptor signaling-mediated cell death by deubiquitinating TRAF6 and IRF3. Knocking down USP4 can enhance T cell infiltration and overcome immune checkpoint blockade resistance [13].
Given the crucial role of DUBs in TME, the development of drugs targeting DUBs has become a potential direction for anti-tumor therapy. For example, USP7 inhibitor, P22077, and USP14 inhibitor, IU1, have shown the potential to inhibit TME pro-tumor function in multiple tumor models [73]. In addition, by combining DUBs targeting with inhibitors of other TME components, such as, immune checkpoint inhibitors, the therapeutic effect may be further improved.
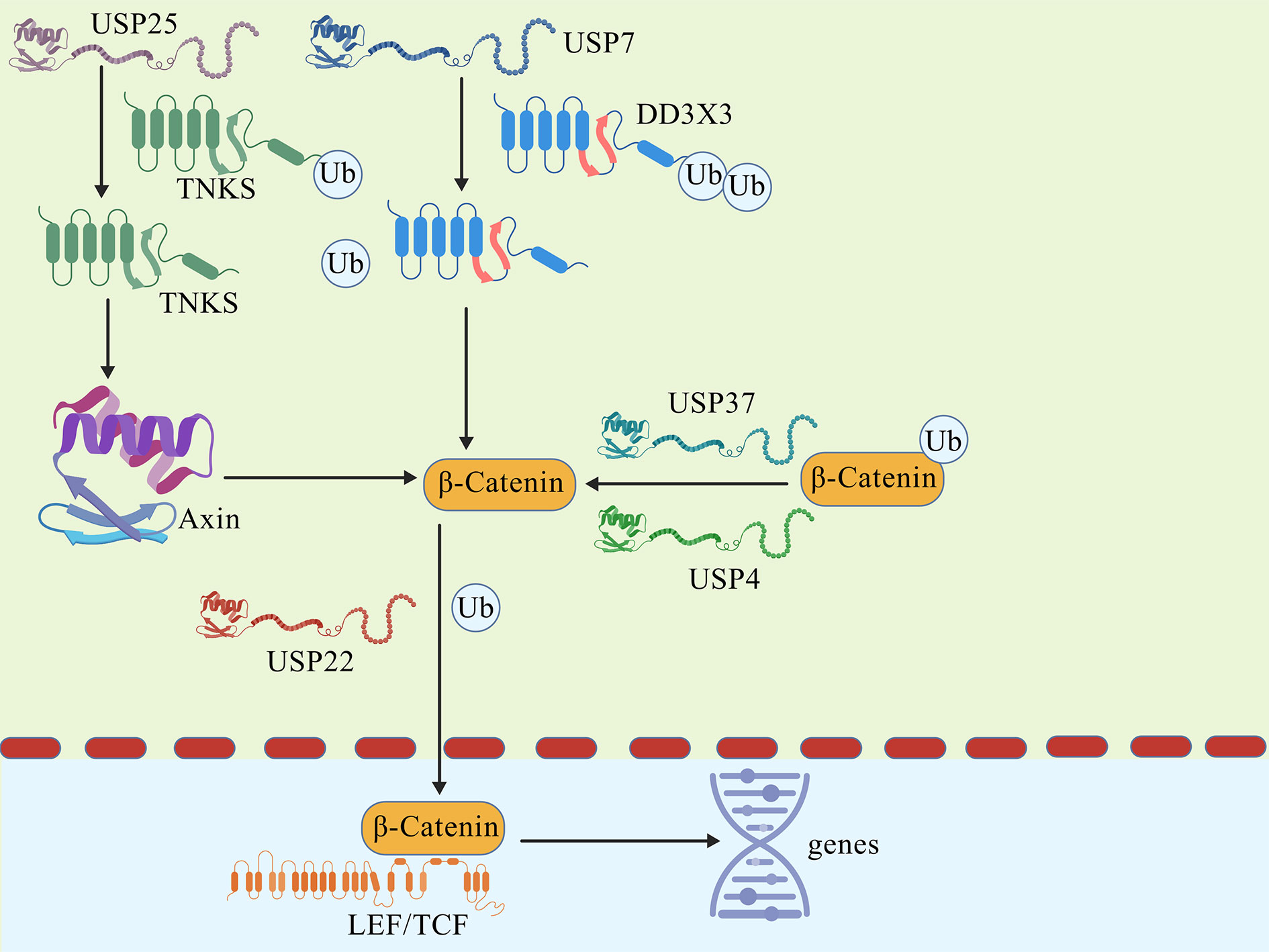 Figure 2. DUBs affect the quantity of β-catenin and the transcription of target genes.
Figure 2. DUBs affect the quantity of β-catenin and the transcription of target genes.
TME plays a crucial role in the occurrence and development of CRC, and DUBs profoundly affect the function of TME by regulating immune-related signaling pathways. For example, USP4 weakens tumor immune response by inhibiting the nuclear localization of IRF3, leading to immune escape in CRC [13]. In addition, USP10 promotes polarization of tumor associated macrophages by stabilizing NLRP7, further exacerbating the invasion and metastasis of CRC [14]. These findings reveal the key role of DUBs in regulating TME immune escape, providing new ideas for targeting DUBs to improve anti-tumor immunotherapy.
Chemotherapy resistance is a significant obstacle in the treatment of CRC and DUBs play a central role in this process. For example, USP9X stabilizes the Mcl-1, an anti-apoptotic protein, inhibits chemotherapy induced apoptosis, and leads to chemotherapy resistance [65, 66]. In addition, USP14 regulates P-glycoprotein expression, promotes drug efflux, and significantly reduces intracellular chemotherapy drug concentration. These research findings suggest that targeting DUBs may be an effective strategy to overcome chemotherapy resistance.
At present, specific inhibitors targeting DUBs have shown significant anti-tumor potential in preclinical studies. For example, USP7 inhibitor P5091 and USP14 inhibitor IU1 showed good anti-tumor effects in the CRC model. However, these inhibitors still face challenges in terms of selectivity and pharmacokinetic properties. In addition, inhibition of DUBs may cause non-specific toxic side effects, limiting their clinical application. Therefore, future research needs to focus on improving the selectivity and safety of DUBs inhibitors, exploring the clinical feasibility of combination with immunotherapy or targeted therapy. Although the important role of DUBs in CRC has gradually been revealed, their molecular mechanisms and regulatory networks still need further in-depth research. Future research directions should include (1) revealing the specific functions of DUBs in different subtypes of CRC; (2) developing efficient and specific DUBs inhibitors, and evaluating their clinical efficacy; (3) exploring the combinationof DUBs with TME targeting and immunotherapy. In summary, DUBs have significant clinical application value as potential targets for CRC treatment. By conducting in-depth research on the mechanism of action of DUBs and optimizing targeted treatment strategies against DUBs, it is expected to provide more precise and efficient treatment plans for CRC patients.
|
Table 1. The role of DUBs in colorectal cancer. |
||
|
DUBs |
Effect |
References |
|
USP1 |
Gene knockout increases the level of phosphorylated p53 and downregulates the levels of Cy cyclin Al, Dl, and El |
[53] |
|
USP2 |
Cell Cycle Protein D1 and PD-L1 Stabilization |
[74] |
|
USP9X |
Stabilization of FBW7 |
[75] |
|
USP29 |
Deletion activates the expression of y-H2AX |
[59] |
|
USP47 |
Promote EMT, Stabilize TCEA3 and regulate YAP protein stability |
[74, 75] |
|
UCHL1 |
Stabilize β-catenin directly |
[76] |
|
UCHL3 |
Promote EMT |
[77] |
|
OTUB1 |
Promote EMT |
[78] |
|
OTUB2 |
Inhibit PKM2 ubiquitination and promote glycolysis |
[12] |
|
PSMD14 |
Stabilize ALK2 and promote efflux of anticancer drugs |
[79] |
|
USP20 |
Directly stabilizing β- catenin protein and participating in immune regulation |
[82, 83] |
|
USP25 |
The increase in TNKS expression negatively regulates the level of Axin protein, thereby enhancing the accumulation of β - catenin |
[84] |
No applicable.
Ethics approval
No applicable.
Data availability
The data will be available upon request.
Funding
None.
Authors’ contribution
Noor Al Shukri contributed to the conception, design, writing of this review article and figures drawing. Razik Bin Abdul Momin revised the review and submitted the final version of the manuscript.
Competing interests
The authors declare no competing interests.
- Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F: Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 2023, 72(2): 338-344.
- Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol 2022, 7(7): 627-647.
- Koroukian SM, Booker BD, Vu L, Schumacher FR, Rose J, Cooper GS, Selfridge JE, Markt SC: Receipt of Targeted Therapy and Survival Outcomes in Patients With Metastatic Colorectal Cancer. JAMA Net Open 2023, 6(1): e2250030-e2250030.
- Rumpold H, Niedersüß-Beke D, Heiler C, Falch D, Wundsam HV, Metz-Gercek S, Piringer G, Thaler J: Prediction of mortality in metastatic colorectal cancer in a real-life population: a multicenter explorative analysis. BMC Cancer 2020, 20(1): 1149.
- Wu Y, Chen Y, Tian X, Shao G, Lin Q, Sun A: Ubiquitination regulates autophagy in cancer: simple modifications, promising targets. J Transl Med 2024, 22(1): 985.
- Niu K, Shi Y, Lv Q, Wang Y, Chen J, Zhang W, Feng K, Zhang Y: Spotlights on ubiquitin-specific protease 12 (USP12) in diseases: from multifaceted roles to pathophysiological mechanisms. J Transl Med 2023, 21(1): 665.
- Han J, Fang Z, Han B, Ye B, Lin W, Jiang Y, Han X, Wang X, Wu G, Wang Y, Liang G: Deubiquitinase JOSD2 improves calcium handling and attenuates cardiac hypertrophy and dysfunction by stabilizing SERCA2a in cardiomyocytes. Nat Cardiovasc Res 2023, 2(8): 764-777.
- Sun T, Liu Z, Yang Q: The role of ubiquitination and deubiquitination in cancer metabolism. Mol Cancer 2020, 19(1): 146.
- Kim S-Y, Baek K-H: TGF-β signaling pathway mediated by deubiquitinating enzymes. Cell Mol Life Sci 2019, 76(4): 653-665.
- Estavoyer B, Messmer C, Echbicheb M, Rudd CE, Milot E, Affar EB: Mechanisms orchestrating the enzymatic activity and cellular functions of deubiquitinases. J Biol Chem 2022, 298(8): 102198.
- Li S, Song Y, Wang K, Liu G, Dong X, Yang F, Chen G, Cao C, Zhang H, Wang M et al: USP32 deubiquitinase: cellular functions, regulatory mechanisms, and potential as a cancer therapy target. Cell Death Discov 2023, 9(1): 338.
- Yu S, Zang W, Qiu Y, Liao L, Zheng X: Deubiquitinase OTUB2 exacerbates the progression of colorectal cancer by promoting PKM2 activity and glycolysis. Oncogene 2022, 41(1): 46-56.
- Zhou Y, Li H, Zhang Y, Zhao E, Huang C, Pan X, Shu F, Liu Z, Tang N, Li F, Liao W: Deubiquitinase USP4 suppresses antitumor immunity by inhibiting IRF3 activation and tumor cell-intrinsic interferon response in colorectal cancer. Cancer Lett 2024, 589: 216836.
- Li B, Qi ZP, He DL, Chen ZH, Liu JY, Wong MW, Zhang JW, Xu EP, Shi Q, Cai SL et al: NLRP7 deubiquitination by USP10 promotes tumor progression and tumor-associated macrophage polarization in colorectal cancer. J Exp Clin Cancer Res 2021, 40(1): 126.
- Kapuria V, Peterson LF, Fang D, Bornmann WG, Talpaz M, Donato NJ: Deubiquitinase Inhibition by Small-Molecule WP1130 Triggers Aggresome Formation and Tumor Cell Apoptosis. Cancer Res 2010, 70(22): 9265-9276.
- Zhang H, Liu W, Wu Y, Chen C: USP3: Key deubiquitylation enzyme in human diseases. Cancer Sci 2024, 115(7): 2094-2106.
- Xu Z, Zhang N, Shi L: Potential roles of UCH family deubiquitinases in tumorigenesis and chemical inhibitors developed against them. Am J Cancer Res 2024, 14(6): 2666-2694.
- Pan X, Wu S, Wei W, Chen Z, Wu Y, Gong K: Structural and Functional Basis of JAMM Deubiquitinating Enzymes in Disease. Biomolecules 2022, 12(7): 910.
- Al-Eidan A, Draper B, Wang S, Coke B, Skipp P, Wang Y, Ewing RM: Knockdown proteomics reveals USP7 as a regulator of cell-cell adhesion in colorectal cancer via AJUBA. Mol Cell Proteomics 2024, 23(12): 100878.
- Zhang N, Wang F, Zhang G, Zhang Q, Liu Y, Wang Q, Elsharkawy MS, Zheng M, Wen J, Zhao G, Li Q: USP7 Promotes deubiquitination and stabilization of MyD88 to enhance immune responses. Front Immunol 2022, 13: 900243.
- Shao Z, Yang W, Qiu H, He Z-H, Lu M, Shen Q, Ding J, Zheng J, Bai JJCD, Disease: The role of USP7-YY1 interaction in promoting colorectal cancer growth and metastasis. Cell Death Dis 2024, 15(5): 347.
- Cao P, Li Q, Zou D, Wang L, Wang Z: Identification of crucial ubiquitin-associated genes for predicting the effects of immunotherapy and therapeutic agents in colorectal cancer. Gene 2024, 904: 148215.
- Kubaichuk K, Seitz T, Bergmann U, Glumoff V, Mennerich D, Kietzmann T: Ubiquitin-specific protease 10 determines colorectal cancer outcome by modulating epidermal growth factor signaling via inositol polyphosphate-4-phosphatase type IIB. Oncogenesis 2024, 13(1): 37.
- Ye Z, Chen J, Huang P, Xuan Z, Zheng S: Ubiquitin-specific peptidase 10, a deubiquitinating enzyme: Assessing its role in tumor prognosis and immune response. Front Oncol 2022, 12: 990195.
- Liu Y, Yang Y, Xu H, Dong X: Implication of USP22 in the regulation of BMI-1, c-Myc, p16INK4a, p14ARF, and cyclin D2 expression in primary colorectal carcinomas. Diagn Mol Pathol 2010, 19(4): 194-200.
- Li Y, Yang Y, Li J, Liu H, Chen F, Li B, Cui B, Liu Y: USP22 drives colorectal cancer invasion and metastasis via epithelial-mesenchymal transition by activating AP4. Oncotarget 2017, 8(20): 32683-32695.
- Lee KC, Chen HH, Cheng KC, Liu TT, Lee KF, Teng CC, Huang CY, Hsieh MC, Kuo HC: Use of iTRAQ-based quantitative proteomic identification of CHGA and UCHL1 correlated with lymph node metastasis in colorectal carcinoma. J Cell Mol Med 2023, 27(14): 2004-2020.
- Ma Y, Zhao M, Zhong J, Shi L, Luo Q, Liu J, Wang J, Yuan X, Huang C: Proteomic profiling of proteins associated with lymph node metastasis in colorectal cancer. J Cell Biochem 2010, 110(6): 1512-1519.
- Fang Y, Shen X: Ubiquitin carboxyl-terminal hydrolases: involvement in cancer progression and clinical implications. Cancer Metastasis Rev 2017, 36(4): 669-682.
- Nam MJ, Madoz-Gurpide J, Wang H, Lescure P, Schmalbach CE, Zhao R, Misek DE, Kuick R, Brenner DE, Hanash SM: Molecular profiling of the immune response in colon cancer using protein microarrays: occurrence of autoantibodies to ubiquitin C-terminal hydrolase L3. Proteomics 2003, 3(11): 2108-2115.
- Wu Q, Huang Y, Gu L, Chang Z, Li G-M: OTUB1 stabilizes mismatch repair protein MSH2 by blocking ubiquitination. J Biol Chem 2021, 296: 100466.
- Zhu D, Xu R, Huang X, Tang Z, Tian Y, Zhang J, Zheng X: Deubiquitinating enzyme OTUB1 promotes cancer cell immunosuppression via preventing ER-associated degradation of immune checkpoint protein PD-L1. Cell Death Differ 2021, 28(6): 1773-1789.
- Wang D, Li Y, Chang W, Feng M, Yang Y, Zhu X, Liu Z, Fu Y: CircSEC24B activates autophagy and induces chemoresistance of colorectal cancer via OTUB1-mediated deubiquitination of SRPX2. Cell Death Dis 2024, 15(9): 693.
- Zhu W, Wu C, Liu Z, Zhao S, Huang J: OTU deubiquitinase, ubiquitin aldehyde binding 2 (OTUB2) modulates the stemness feature, chemoresistance, and epithelial-mesenchymal transition of colon cancer via regulating GINS complex subunit 1 (GINS1) expression. Cell Commun Signal 2024, 22(1): 420.
- Chen Y, Shen H, Wang Z, Huang C, Zhang H, Shao Y, Tong Y, Xu L, Lu Y, Fu Z: Recruitment of USP10 by GCS1 to deubiquitinate GRP78 promotes the progression of colorectal cancer via alleviating endoplasmic reticulum stress. J Exp Clin Cancer Res 2024, 43(1): 261.
- Ng H-M, Wei L, Lan L, Huen MSY: The Lys63-deubiquitylating Enzyme BRCC36 Limits DNA Break Processing and Repair. J Biol Chem 2016, 291(31): 16197-16207.
- Damgaard RB: The ubiquitin system: from cell signalling to disease biology and new therapeutic opportunities. Cell Death Differ 2021, 28(2): 423-426.
- Liao Y, Zhang W, Liu Y, Zhu C, Zou Z: The role of ubiquitination in health and disease. MedComm (2020) 2024, 5(10): e736.
- Snyder NA, Silva GM: Deubiquitinating enzymes (DUBs): Regulation, homeostasis, and oxidative stress response. J Biol Chem 2021, 297(3): 101077.
- Dai X, Zhang T, Hua D: Ubiquitination and SUMOylation: protein homeostasis control over cancer. Epigenomics 2022, 14(1): 43-58.
- Zhang X, Linder S, Bazzaro M: Drug Development Targeting the Ubiquitin-Proteasome System (UPS) for the Treatment of Human Cancers. Cancers (Basel) 2020, 12(4): 902.
- Kerscher O, Felberbaum R, Hochstrasser M: Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol 2006, 22: 159-180.
- Pohl C, Dikic I: Cellular quality control by the ubiquitin-proteasome system and autophagy. Science 2019, 366(6467): 818-822.
- Tracz M, Bialek W: Beyond K48 and K63: non-canonical protein ubiquitination. Cell Mol Biol Lett 2021, 26(1): 1.
- Grice GL, Nathan JA: The recognition of ubiquitinated proteins by the proteasome. Cell Mol Life Sci 2016, 73(18): 3497-3506.
- Park J, Cho J, Song EJ: Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch Pharm Res 2020, 43(11): 1144-1161.
- Wei R, Liu X, Yu W, Yang T, Cai W, Liu J, Huang X, Xu GT, Zhao S, Yang J, Liu S: Deubiquitinases in cancer. Oncotarget 2015, 6(15):12872-12889.
- Pfoh R, Lacdao IK, Saridakis V: Deubiquitinases and the new therapeutic opportunities offered to cancer. Endocr Relat Cancer 2015, 22(1): T35-54.
- Reissland M, Hartmann O, Tauch S, Bugter JM, Prieto-Garcia C, Schulte C, Loebbert S, Solvie D, Bitman-Lotan E, Narain A et al: USP10 drives cancer stemness and enables super-competitor signalling in colorectal cancer. Oncogene 2024, 43(50): 3645-3659.
- Kumar S, Basu M, Ghosh MK: E3 ubiquitin ligases and deubiquitinases in colorectal cancer: Emerging molecular insights and therapeutic opportunities. Biochim Biophys Acta Mol Cell Res 2024, 1871(8): 119827.
- Almalki SG: The pathophysiology of the cell cycle in cancer and treatment strategies using various cell cycle checkpoint inhibitors. Pathol Res Pract 2023, 251: 154854.
- Zhang H, Lin J, Zheng S, Ma L, Pang Z, Yin H, Meng C, Wang Y, Han Q, Zhang X et al: CDKL3 is a targetable regulator of cell cycle progression in cancers. J Clin Invest 2024, 134(16): e178428.
- Xu X, Li S, Cui X, Han K, Wang J, Hou X, Cui L, He S, Xiao J, Yang Y: Inhibition of Ubiquitin Specific Protease 1 Sensitizes Colorectal Cancer Cells to DNA-Damaging Chemotherapeutics. Front Oncol 2019, 9: 1406.
- Montalto FI, Amicis FDJC: Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9(12): 2648.
- Shan J, Zhao W, Gu WJMC: Suppression of Cancer Cell Growth by Promoting Cyclin D1 Degradation. Mol Cell 2009, 36(3): 469-476.
- Xu X, Huang A, Cui X, Han K, Hou X, Wang Q, Cui L, Yang Y: Ubiquitin specific peptidase 5 regulates colorectal cancer cell growth by stabilizing Tu translation elongation factor. Theranostics 2019, 9(14): 4208-4220.
- Becker K, Marchenko ND, Palacios G, Moll UM: A role of HAUSP in tumor suppression in a human colon carcinoma xenograft model. Cell Cycle 2008, 7(9): 1205-1213.
- Dai X, Lu L, Deng S, Meng J, Wan C, Huang J, Sun Y, Hu Y, Wu B, Wu G et al: USP7 targeting modulates anti-tumor immune response by reprogramming Tumor-associated Macrophages in Lung Cancer. Theranostics 2020, 10(20): 9332-9347.
- Chandrasekaran AP, Suresh B, Sarodaya N, Ko NR, Oh SJ, Kim KS, Ramakrishna S: Ubiquitin Specific Protease 29 Functions as an Oncogene Promoting Tumorigenesis in Colorectal Carcinoma. Cancers (Basel) 2021, 13(11): 2706.
- Hamilton E, Infante JR: Targeting CDK4/6 in patients with cancer. Cancer Treat Rev 2016, 45: 129-138.
- Tong D, Yuan J, Wang Z, Yu E, E J: USP37 promotes angiogenesis and metastasis in colorectal cancer by facilitating β-catenin stability. Am J Cancer Res 2023, 13(6): 2323-2341.
- Li Y, Wang X: Deubiquitinating enzymes for targeted cancer therapy. Clin Transl Discov 2023, 3(6): e256.
- Sun H, Ou B, Zhao S, Liu X, Song L, Liu X, Wang R, Peng Z: USP11 promotes growth and metastasis of colorectal cancer via PPP1CA-mediated activation of ERK/MAPK signaling pathway. EBioMedicine 2019, 48: 236-247.
- Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, Zhou Z, Shu G, Yin G: Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther 2022, 7(1): 3.
- Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, Maecker H, O'Rourke K, Bazan F, Eastham-Anderson J et al: Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 2010, 463(7277): 103-107.
- Harris DR, Mims A, Bunz F: Genetic disruption of USP9X sensitizes colorectal cancer cells to 5-fluorouracil. Cancer Biol Ther 2012, 13(13): 1319-1324.
- Cao Y, Tang H, Wang G, Li P, Song Z, Li W, Sun X, Zhong X, Yu Q, Zhu S, Zhu L: Targeting survivin with Tanshinone IIA inhibits tumor growth and overcomes chemoresistance in colorectal cancer. Cell Death Discov 2023, 9(1): 351.
- Tanguturi P, Kim K-S, Ramakrishna S: The role of deubiquitinating enzymes in cancer drug resistance. Cancer Chemother Pharmacol 2020, 85(4): 627-639.
- Chuang L, Qifeng J, Shaolei Y: The tumor immune microenvironment and T-cell-related immunotherapies in colorectal cancer. Discov Oncol 2024, 15(1): 244.
- Guo X-w, Lei R-e, Zhou Q-n, Zhang G, Hu B-l, Liang Y-x: Tumor microenvironment characterization in colorectal cancer to identify prognostic and immunotherapy genes signature. BMC Cancer 2023, 23(1): 773.
- Wu X, Yan H, Qiu M, Qu X, Wang J, Xu S, Zheng Y, Ge M, Yan L, Liang L: Comprehensive characterization of tumor microenvironment in colorectal cancer via molecular analysis. eLife 2023, 12: e86032.
- Wang XM, Yang C, Zhao Y, Xu ZG, Yang W, Wang P, Lin D, Xiong B, Fang JY, Dong C, Zhong B: The deubiquitinase USP25 supports colonic inflammation and bacterial infection and promotes colorectal cancer. Nat Cancer 2020, 1(8): 811-825.
- Gao H, Yin J, Ji C, Yu X, Xue J, Guan X, Zhang S, Liu X, Xing F: Targeting ubiquitin specific proteases (USPs) in cancer immunotherapy: from basic research to preclinical application. J Exp Clin Cancer Res 2023, 42(1): 225.
- Shan J, Zhao W, Gu W: Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol Cell 2009, 36(3): 469-476.
- Khan OM, Carvalho J, Spencer-Dene B, Mitter R, Frith D, Snijders AP, Wood SA, Behrens A: The deubiquitinase USP9X regulates FBW7 stability and suppresses colorectal cancer. J Clin Invest 2018, 128(4): 1326-1337.
- Choi BJ, Park SA, Lee SY, Cha YN, Surh YJ: Hypoxia induces epithelial-mesenchymal transition in colorectal cancer cells through ubiquitin-specific protease 47-mediated stabilization of Snail: A potential role of Sox9. Sci Rep 2017, 7(1): 15918.
- Hou X, Xia J, Feng Y, Cui L, Yang Y, Yang P, Xu X: USP47-Mediated Deubiquitination and Stabilization of TCEA3 Attenuates Pyroptosis and Apoptosis of Colorectal Cancer Cells Induced by Chemotherapeutic Doxorubicin. Front Pharmacol 2021, 12: 713322.
- Zhong J, Zhao M, Ma Y, Luo Q, Liu J, Wang J, Yuan X, Sang J, Huang C: UCHL1 acts as a colorectal cancer oncogene via activation of the β-catenin/TCF pathway through its deubiquitinating activity. Int J Mol Med 2012, 30(2): 430-436.
- Li J, Zheng Y, Li X, Dong X, Chen W, Guan Z, Zhang C: UCHL3 promotes proliferation of colorectal cancer cells by regulating SOX12 via AKT/mTOR signaling pathway. Am J Transl Res 2020, 12(10): 6445-6454.
- Zhou Y, Wu J, Fu X, Du W, Zhou L, Meng X, Yu H, Lin J, Ye W, Liu J et al: OTUB1 promotes metastasis and serves as a marker of poor prognosis in colorectal cancer. Mol Cancer 2014, 13: 258.
- Seo D, Jung SM, Park JS, Lee J, Ha J, Kim M, Park SH: The deubiquitinating enzyme PSMD14 facilitates tumor growth and chemoresistance through stabilizing the ALK2 receptor in the initiation of BMP6 signaling pathway. EBioMedicine 2019, 49: 55-71.
- Wu C, Luo K, Zhao F, Yin P, Song Y, Deng M, Huang J, Chen Y, Li L, Lee S et al: USP20 positively regulates tumorigenesis and chemoresistance through β-catenin stabilization. Cell Death Differ 2018, 25(10): 1855-1869.
- Jin R, Luo Z, Jun L, Tao Q, Wang P, Cai X, Jiang L, Zeng C, Chen Y: USP20 is a predictor of poor prognosis in colorectal cancer and associated with lymph node metastasis, immune infiltration and chemotherapy resistance. Front Oncol 2023, 13: 1023292.
- Xu D, Liu J, Fu T, Shan B, Qian L, Pan L, Yuan J: USP25 regulates Wnt signaling by controlling the stability of tankyrases. Genes Dev 2017, 31(10): 1024-1035.
Asia-Pacific Journal of Oncology
print ISSN: 2708-7980, online ISSN: 2708-7999
 Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript