Research Article | Open Access
Associations between KRAS status and clinical features in non-polyp colon cancer patients
Cuong Hoang Minh1, Huong Bui Thi Thu2, Loi Nguyen Thuan3, Thang Vu Hong1
1Oncology Department, Hanoi Medical University, Hanoi, Vietnam.
2Department of Biochemistry, Thai Nguyen University of Medicine and Pharmacy, Thai Nguyen, Vietnam.
3Nuclear Medicine and Oncology Center, Bach Mai Hospital, Hanoi, Vietnam.
Correspondence: Thang Vu Hong (Oncology Department, Hanoi Medical University, Hanoi, 100000, Vietnam; Email: vuhongthang@hmu.edu.vn).
Asia-Pacific Journal of Oncology 2023, 4: 10-16. https://doi.org/10.32948/ajo.2023.06.28
Received: 10 May 2023 | Accepted: 26 Jun 2023 | Published online: 29 Jun 2023
Methods Patients with NPCC (stages II or III) and without historical or current polyp appearance were included. Genomic DNA samples were prepared from dissected tumors, and specific sequences of the KRAS gene were amplified by PCR. The mutations at codons 12, 13, 59, 60, 61, 117, and 146 of the gene were determined by using a commercial kit. Possible associations of the KRAS mutation with clinicopathological properties were analyzed using SPSS and GraphPad Prism.
Results The KRAS mutation rate was 47.9% in NPCC patients; mutations in exon 2 accounted for 91.4% of all detected mutations. Moreover, the KRAS mutation rate was higher in females (57.1%) than in males (39.8%). The association of KRAS mutation with female NPCC patients was further confirmed by multivariate regression data with OR=2.144 and p = 0.012.
Conclusion The KRAS mutation rate was also higher in patients with right colon cancers. The mutated-KRAS-carrying patients potentially experienced anemia. The data provide important scientific background for the treatment and management of the disease.
Keywords non-polyp colon cancer, KRAS mutation in NPCC, non-polyp colon cancer, stage II-III
CC is the fourth deadliest cancer in Vietnam (both genders combined) [19], with an increasing trend of new cases [20]. Most Vietnamese patients were diagnosed in stages II or III (by AJCC 8th) since the tumors invaded muscle layers or migrated to local nodes [16]. However, only a few studies on KRAS mutations in Vietnamese colorectal cancer, with most of the recruited patients carrying the CC, have been reported [21, 22]. The KRAS status of only non-polyp colon cancer (NPCC) has not been elucidated. Furthermore, the association of the KRAS status with the clinical and clinicopathological properties of the Vietnamese NPCC is also under establishment. Therefore, this study aims to determine KRAS mutations and their associations with clinicopathological features in Vietnamese patients who were hospitalized for surgery to remove NPCC.
Data in this study covered all patients with non-polyp colon cancer stages II or III who were hospitalized from January 2016 to August 2020 at Bach Mai, Viet Duc, and the Vietnam National Cancer Hospital—the three biggest national hospitals in northern Vietnam. Patients with colon cancer and without tumors in any other tissues or organs are included. The stage of colon cancer (II or III) was followed by the standards of the American Joint Committee on Cancer (AJCC), version 8. The term "non-polyp" described patients without a historical or current polyp appearance. Non-polyp status was examined by colonoscopy and/or confirmed by observing the dissected tissues after radical surgery. Patients with the appearance of colon polyps in any number at any time were excluded.
The Ethics Committee of Hanoi Medical University approved the study. The patients consented to participate in the study. Collecting and using personal information and clinicopathological characteristics was performed with the permission of the patients. The handling of the collected information, which was classified and safely stored as confidential, was strictly limited to assigned members.
DNA isolation and Mutation analysis
Investigation of KRAS mutations was performed with formalin-fixed, paraffin-embedded (FFPE) tissue. Five 10-m-thick tissue slides from each FFPE tissue block (194 samples) were used for the isolation of genomic DNA. Paraffin in FFPE tissue slides was removed by using FFPE deparaffinization solution (MERCK, Germany), and DNA was then extracted from tissue samples by using the PureLinkTM Genomic DNA Mini Kit (Invitrogen, USA).
KRAS mutations were detected by using the KRAS XL StripAssay (ViennaLab, Austria) in a five-step procedure including amplification, hybridization, stringent wash, and color development. Briefly, prepared DNA samples were mixed with Taq polymerase and an amplification mixture from the manufacturer. The specific DNA sequences were amplified in 35 cycles of polymerase chain reactions (PCR). The PCR products were then denatured and hybridized with probes on strips in the hybridization buffer. After hybridization ended, solutions were removed and the strips were washed. A conjugated solution was then added to the strips, followed by a second washing step. At last, color developer was added to the strips in the dark for the appearance of purple positive bands, and then a third washing step took place. The strips were analyzed to determine KRAS mutations at codons 12, 13, 59, 60, 61, 117, and 146.
Statistics
The KRAS XL StripAssay (ViennaLab, Austria) used a five-step procedure that included amplification, hybridization, stringent wash, and color development to find KRAS mutations. Briefly, prepared DNA samples were mixed with Taq polymerase and an amplification mixture from the manufacturer. The specific DNA sequences were amplified in 35 cycles of polymerase chain reactions (PCR). The PCR products were then denatured and hybridized with probes on strips in the hybridization buffer. After hybridization ended, solutions were removed and the strips were washed. The strips then received a conjugated solution, and a second washing process followed. At last, color developer was added to the strips in the dark for the appearance of purple positive bands, and then a third washing step took place. The strips were analyzed to determine KRAS mutations at codons 12, 13, 59, 60, 61, 117, and 146.
During the course of the study, a total of 194 patients who satisfied the selection criteria were included in the analysis. Among them, 91 (46.9%) were females, of whom 39 (20.1%) carried the wild-type and 52 (26.8%) the mutated KRAS. On the other hand, 103 (53.1%) were males, including 62 (32.0%) wild-type and 41 (21.1%) mutated KRAS. The age of the patients ranged from 24 to 75, with an average of 55.4 ± 12.2. Summarized in Figure 1, the minimum ages of the mutated KRAS-carrying males and females were lower than those of the patients with the wild-type KRAS. The average age of the mutated patients was 53.91 (54.51 for the mutated KRAS males; 53.44 for females), which is also lower than 56.77 for the wild-type KRAS group (57.37 for males; 55.82 for females) (Figure 1). However, pair-wise comparisons of the average ages showed no significant changes in age between the mutated and the normal groups (data not shown).
Mutations in KRAS gene in Vietnamese NPCC stages II and III
Point mutations of KRAS in exons 2 (codons 12 and 13), 3 (codons 59 and 61), and 4 (codons 117 and 146) were determined. The overall KRAS mutation rate was 47.9% in patients (Figure 2). Of which, mutations in exon 2 were absolutely dominant, with 85 cases accounting for 43.8% of recruited patients (or 91.4% of total detected mutations). Mutations in exon 3, including 1 case at codon 59 and 5 cases at codon 61, resulted in 3.1%. Only two cases of mutated exon 4 were detected (1%); one carried a mutation at codon 117 and the other at codon 146. None of the patients were found to carry more than one mutation (Figure 2).
Associations of KRAS status and clinical features
A pairwise comparison between the wild-type and mutated KRAS groups for each symptom was brought about to identify a possible association of a symptom with the KRAS status. Most of the observed symptoms were unlikely to correlate with the KRAS mutations (Figure 3). Abdominal pains were highly recorded in both groups, with 84.2% and 77.4% in the wide-type group and mutation groups, respectively. Other symptoms that neither showed considerable changes between the two groups included bleeding (14.9% vs. 22.6%), blood in stool (21.8% vs. 11.8%), diarrhea (25.7% vs. 26.9%), constipation (19.8% vs. 17.2%), and weight loss (19.8% vs. 20.4%). However, the analysis revealed a significant increase in anemia cases among the mutated group (p = 0.041). Particularly, 28.0% of mutated-KRAS patients exhibited anemia symptoms, while only 15.8% of the wide-type group did, indicating an association of the anemia with the KRAS mutation in NPCC (Figure 3).
Association of KRAS mutation and clinicopathological features in patients with NPCC stages II and III
Table 1 demonstrated possible associations of clinicopathological features with KRAS status in patients. Conspicuously, the KRAS mutation rate in females was statistically higher than in males. With 52 mutated females, the KRAS mutation rate was 55.9% of the cases, far higher than the 44.1% contributed by the males (Table 1). The mutations were also detected at a higher rate in tumors in the right colon compared with the left colon (54.8% right colons vs. 45.2% left colons, p = 0.047) (Table 1). In contrast, KRAS mutation rates were relatively compatible among younger and older patients (Table 1).
Histologic examination showed a high rate of adenocarcinoma (AC) with 164 confirmed cases (84.5% of patients), while other types were identified in 30 cases (15.5% of patients) (Table 1). The numbers of NPCC AC cases in mutated vs. wild-type KRAS were almost identical, 80 and 84, respectively, indicating that the KRAS mutation is unlikely to cause the AC. The KRAS mutation rate among the AC was also not different from that among the other types.
The invasion depth of tumors in Vietnamese NPCC was likely linked to the KRAS mutation. The T4 stage (which included T4a and b) was confirmed in 59 cases of the mutated group, accounting for 63.5%. This stage was confirmed in only 54 wild-type patients (53.5% of the wild-type group). Similarly, stage III tumors were determined at a higher rate among the mutated patients (52 out of 93 cases, or 55.9%) compared with the same stage among the wild-type patients (49 out of 101 cases, or 48.5%). However, none of the linkages between the staging and KRAS mutation were statistically significant, including nodal stages (Table 1).
Multivariate regression analysis: interaction of KRAS mutations with other prognostic markers
The association of KRAS mutations with female among Vietnamese NPCC patients was confirmed by multivariate logistic regression. The KRAS mutation rate was significantly higher in females, with OR = 2.144 (95% confidence interval (CI): 1.184–3.882, p = 0.012). The analysis further confirmed the correlation of KRAS mutations with the right NPCC, with p = 0.048. Therein, the odds ratio of left colon tumors to right colon tumors was 0.551 (95% confidence interval (CI): 0.305-0.996) (Table 2). Besides the differences above, there was no evidence of an association between tumor staging and histologic types and KRAS mutations among NPCC in Vietnam.
|
Table 1. Clinicopathological features and KRAS status of tumor-dissected patients. |
|||||
|
Characteristic |
Total (n=194) |
KRAS mutatio status |
P value |
||
|
Wild type N=(101, 52.1%) |
Mutated (n=93, 47.9%) |
||||
|
Age |
< 50 |
56 |
28(27.7%) |
28(30.1%) |
0.714 |
|
≥50 |
138 |
73(72.3%) |
65(69.9%) |
||
|
Gender |
Male |
103 |
62(61.4%) |
41(44.1%) |
0.016* |
|
Female |
91 |
39(38.6%) |
52(55.9%) |
||
|
Tumor location |
Right colon |
92 |
41(40.6%) |
51(54.8%) |
0.047* |
|
Left colon |
102 |
60(59.4%) |
42(45.2%) |
||
|
Histology |
AC |
164 |
84(83.2%) |
80(86.0%) |
0.583 |
|
Others |
30 |
17(16.8%) |
13(14.0%) |
||
|
Invasion depth |
T3 |
81 |
47(46.5%) |
34(36.6%) |
0.333 |
|
T4a |
92 |
43(42.6%) |
49(52.7%) |
||
|
T4b |
21 |
11(10.9%) |
10(10.8%) |
||
|
Nodal stage |
N0 |
93 |
52(51.5%) |
41(44.1%) |
0.190 |
|
N1 |
71 |
32(30.7%) |
40(43.0%) |
||
|
N2 |
30 |
18(17.8%) |
12(12.9%) |
||
|
TNM stage |
II |
93 |
52(51.5%) |
41(44.1%) |
0.303 |
|
III |
101 |
49(48.5%) |
52(55.9%) |
||
|
AC: adenocarcinoma; *: There were statistical significance. |
|||||
|
Table 2. Interactions between KRAS mutation and prognostic markers. |
||||
|
KRAS mutation |
Multivarite logistic regression |
|||
|
OR |
95%CI |
P value |
||
|
Age |
≥50 / < 50 |
0.821 |
0.426 - 1.582 |
0.555 |
|
Gender |
Femal / Male |
2.144 |
1.184 - 3.882 |
0.012 |
|
Tumor location |
Left / Right |
0551 |
0.305 - 0.996 |
0.048 |
|
Invasion depth |
T4 / T3 |
1.421 |
0.776 - 2.600 |
0.255 |
|
Nodal status |
N(+) / (-) |
1.239 |
0.681 - 2.253 |
0.483 |
|
Histology |
AC / Others |
1.726 |
0.740 - 4.026 |
0.206 |
|
OR: odds ratio; AC: Adenocarcinoma. |
||||
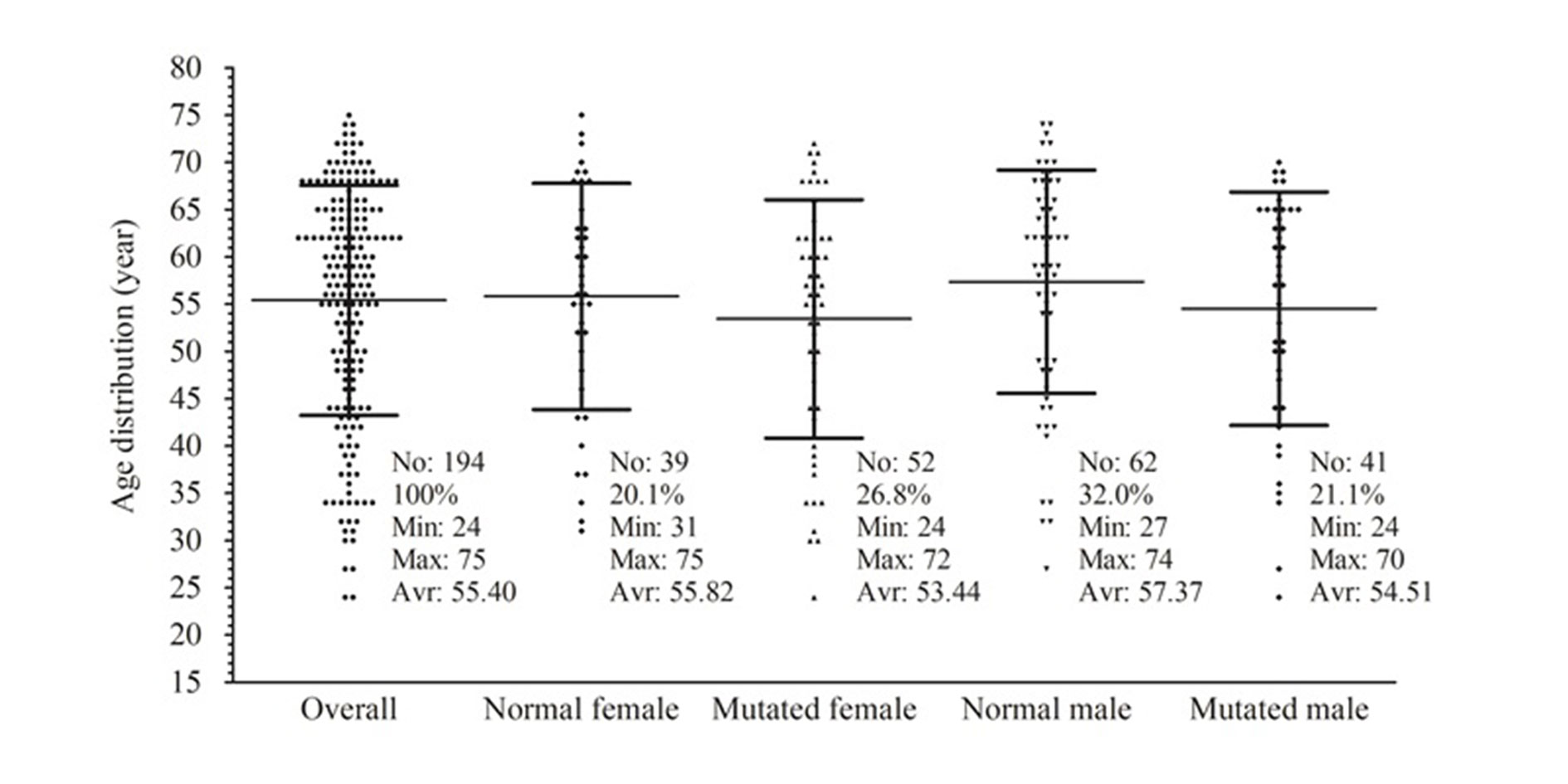 Figure 1. Age distributions by gender in accordance with KRAS status of patients with NPCC.
Figure 1. Age distributions by gender in accordance with KRAS status of patients with NPCC.
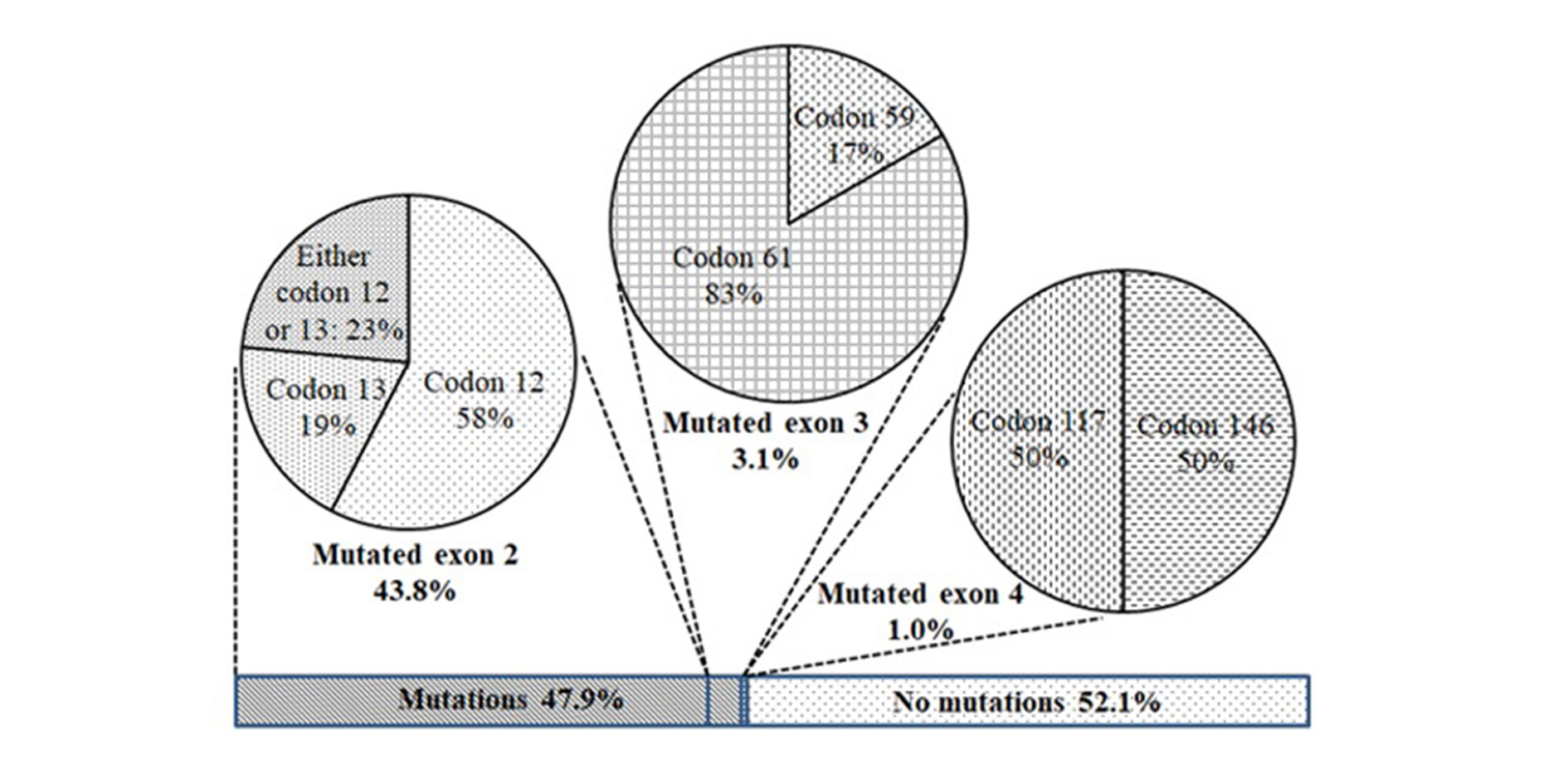 Figure 2. Positions of KRAS mutation in dissected tumors from Vietnamese patients with NPCC.
Figure 2. Positions of KRAS mutation in dissected tumors from Vietnamese patients with NPCC.
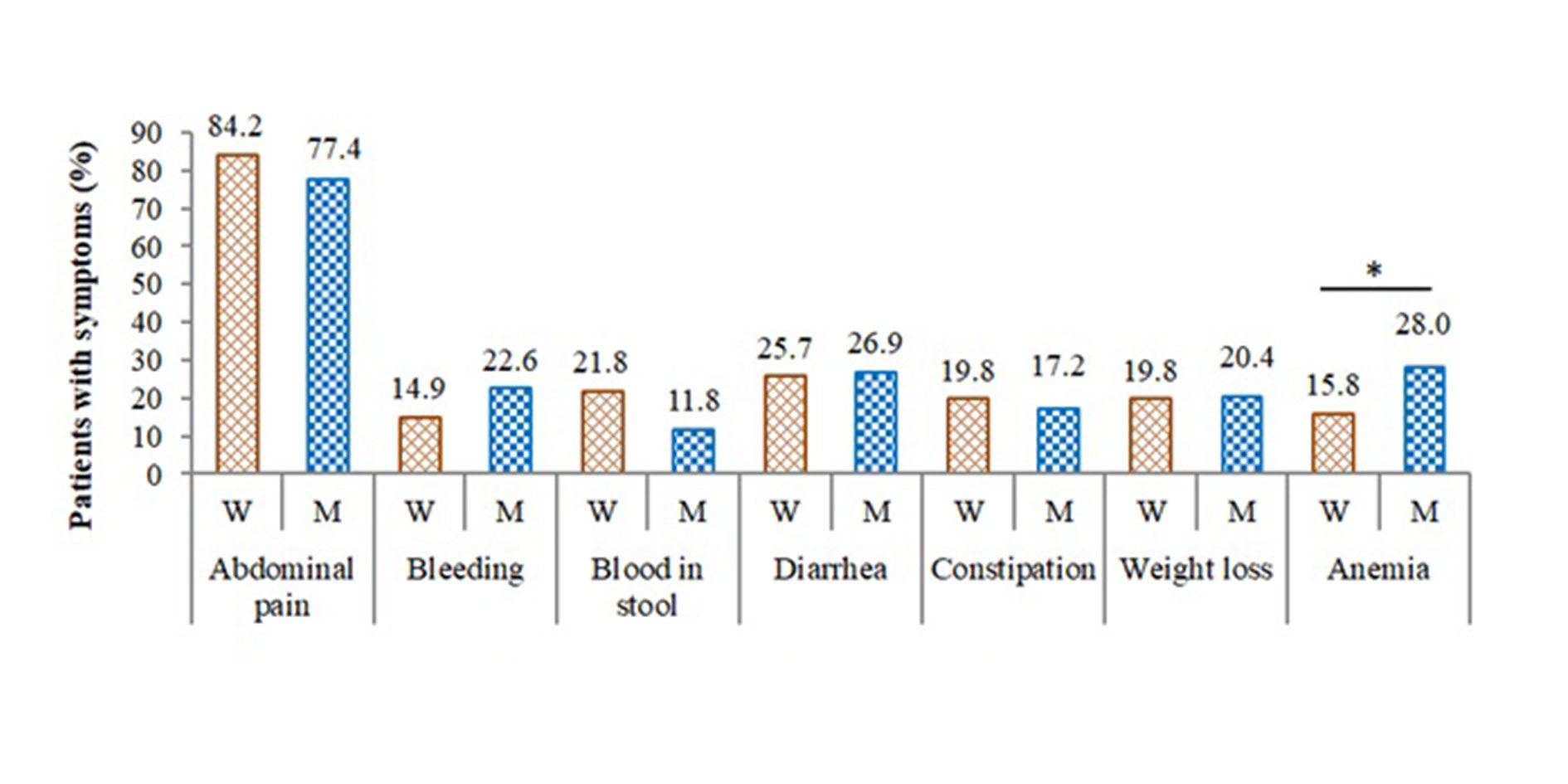 Figure 3. Clinical properties of patients with NPCC before surgery based on KRAS status.
Figure 3. Clinical properties of patients with NPCC before surgery based on KRAS status.
Another impressive finding in this study was the association of KRAS mutations with female patients. The mutations were detected in 57.1% of female patients (52 mutants out of 91 females) and only 39.8% of male patients (41 mutants out of 103 males). Females accounted for 55.9% of mutated patients (Table 1), while males were 44.1%. These gender ratios among mutated patients were contrary to those in a previous study for CRC in Vietnam, which reported 48.2% and 51.8% for mutated females and males, respectively [22]. The contrary suggested an association of KRAS mutations with the NPCC in females, which was further confirmed by our multivariate logistic regression data shown in Table 2. Besides, the age of KRAS mutation-carrying patients in both genders was apparently lower than that of the wild-type groups. However, the correlation of the KRAS mutation with the patient’s age was unclear.
Several reports showed that the KRAS mutation rate was likely higher in the right colon than in the left [3, 18, 26]. In the present study, the linkage of the KRAS mutation rate with right colon tumors was clarified. The KRAS mutation rate in the right colon was 55.4% (51 mutants out of 92), while the rate in the left colon was only 41.2% (42 mutants out of 102). Multivariate regression analysis showed significantly higher KRAS mutations in right colon tumors with = 0.048). The right colon cancers, in comparison with the left ones, were previously reported to be linked with worse survival of the patients [3]. A further study is required to investigate the survival of Vietnamese patients with NPCC.
Similarly, clinicopathological properties such as abdominal pain, anemia, and various symptoms were identified in over 83% of patients. Of those, diarrhea and anemia were likely correlated (close to statistically significant change) to the tumors in the right colon [27]. Logically, a linkage between diarrhea and anemia with KRAS mutations is suspected. However, our current data has confirmed significantly higher anemia cases but not diarrhea cases among KRAS-mutated NPCC patients.
None.
Availability of data and materials
Data and materials are available on request from the authors.
Ethical policy
The study was approved by the Ethics Committee of Hanoi Medical University (Approval number: NCS28/HMU-IRB).
Author contributions
MCH conceptualized, designed, conducted research, and wrote the first draft. TDL, TTHB, TLN, TJA, and HTV provided supervision and revision of the draft.
Competing interests
The authors have no conflicts of interest regarding the publication of this paper.
Funding
This study was funded by the Waikato Medical Research Foundation (#336) and Health Research Council of New Zealand (HRC Ref ID: 21/990).
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021, 71(3): 209-249.
- Alves Martins BA, de Bulhoes GF, Cavalcanti IN, Martins MM, de Oliveira PG, Martins AMA: Biomarkers in colorectal cancer: the role of translational proteomics research. Front Oncol 2019, 9: 1284.
- Tran CG, Goffredo P, Mott SL, Hart A, You YN, Vauthey J-N, Weigel RJ, Hassan I: The impact of KRAS mutation, microsatellite instability, and tumor laterality on the prognosis of nonmetastatic colon cancer. Surgery 2022, 171(3): 657-665.
- Lee D-W, Kim KJ, Han S-W, Lee HJ, Rhee YY, Bae JM, Cho N-Y, Lee K-H, Kim T-Y, Oh D-Y: KRAS mutation is associated with worse prognosis in stage III or high-risk stage II colon cancer patients treated with adjuvant FOLFOX. Ann Surg Oncol 2015, 22: 187-194.
- Jones RP, Sutton PA, Evans JP, Clifford R, McAvoy A, Lewis J, Rousseau A, Mountford R, McWhirter D, Malik HZ: Specific mutations in KRAS codon 12 are associated with worse overall survival in patients with advanced and recurrent colorectal cancer. Br J Cancer 2017, 116(7): 923-929.
- Formica V, Sera F, Cremolini C, Riondino S, Morelli C, Arkenau H-T, Roselli M: KRAS and BRAF mutations in stage II and III colon cancer: a systematic review and meta-analysis. J Natl Cancer Inst 2022, 114(4): 517-527.
- Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y: The RAS/MAPK syndromes: novel roles of the RAS pathway in human genetic disorders. Hum Mutat 2008, 29(8): 992-1006.
- Jin CE, Yeom SS, Koo B, Lee TY, Lee JH, Shin Y, Lim SB: Rapid and accurate detection of KRAS mutations in colorectal cancers using the isothermal-based optical sensor for companion diagnostics. Oncotarget 2017, 8(48): 83860-83871.
- Yoon HH, Tougeron D, Shi Q, Alberts SR, Mahoney MR, Nelson GD, Nair SG, Thibodeau SN, Goldberg RM, Sargent DJ, et al: KRAS codon 12 and 13 mutations in relation to disease-free survival in BRAF-wild-type stage III colon cancers from an adjuvant chemotherapy trial (N0147 alliance). Clin Cancer Res 2014, 20(11): 3033-3043.
- Tong JH, Lung RW, Sin FM, Law PP, Kang W, Chan AW, Ma BB, Mak TW, Ng SS, To KF: Characterization of rare transforming KRAS mutations in sporadic colorectal cancer. Cancer Biol Ther 2014, 15(6): 768-776.
- Sirisena ND, Deen K, Mandawala DEN, Herath P, Dissanayake VHW: The pattern of KRAS mutations in metastatic colorectal cancer: a retrospective audit from Sri Lanka. BMC Res Notes 2017, 10(1): 392.
- Roberts PJ, Der CJ: Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26(22): 3291-3310.
- Krasinskas AM: EGFR Signaling in Colorectal Carcinoma. Patholog Res Int 2011, 2011: 932932.
- Takács T, Kudlik G, Kurilla A, Szeder B, Buday L, Vas V: The effects of mutant Ras proteins on the cell signalome. Cancer Metastasis Rev 2020, 39(4): 1051-1065.
- Nguyen HT, Duong HQ: The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy. Oncol Lett 2018, 16(1): 9-18.
- Weiser MR: AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol 2018, 25(6): 1454-1455.
- Huang L, Guo Z, Wang F, Fu L: KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther 2021, 6(1): 386.
- Scott A, Goffredo P, Ginader T, Hrabe J, Gribovskaja-Rupp I, Kapadia MR, Weigel RJ, Hassan I: The Impact of KRAS Mutation on the Presentation and Prognosis of Non-Metastatic Colon Cancer: an Analysis from the National Cancer Database. J Gastrointest Surg 2020, 24(6): 1402-1410.
- Pham T, Bui L, Kim G, Hoang D, Tran T, Hoang M: Cancers in Vietnam-Burden and Control Efforts: A Narrative Scoping Review. Cancer Control 2019, 26(1): 1073274819863802.
- Pham DX, Phung AHT, Nguyen HD, Bui TD, Mai LD, Tran BNH, Tran TS, Nguyen TV, Ho-Pham LT: Trends in colorectal cancer incidence in Ho Chi Minh City, Vietnam (1996-2015): Joinpoint regression and age-period-cohort analyses. Cancer Epidemiol 2022, 77: 102113.
- Do MD, Nguyen TH, Le KT, Le LHG, Nguyen BH, Le KT, Doan TPT, Ho CQ, Nguyen HN, Tran TD, et al: Molecular characteristics of young-onset colorectal cancer in Vietnamese patients. Asia Pac J Clin Oncol 2022, 18(6): 678-685.
- Nguyen HT, Le DT, Duong QH, Tatipamula VB, Van Nguyen B: High frequency of microsatellite instability and its substantial co-existence with KRAS and BRAF mutations in Vietnamese patients with colorectal cancer. Oncol Lett 2021, 21(1): 41.
- Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C, et al: Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol 2010, 28(3): 466-474.
- Shen Y, Han X, Wang J, Wang S, Yang H, Lu SH, Shi Y: Prognostic impact of mutation profiling in patients with stage II and III colon cancer. Sci Rep 2016, 6: 24310.
- Guo F, Gong H, Zhao H, Chen J, Zhang Y, Zhang L, Shi X, Zhang A, Jin H, Zhang J, et al: Mutation status and prognostic values of KRAS, NRAS, BRAF and PIK3CA in 353 Chinese colorectal cancer patients. Sci Rep 2018, 8(1): 6076.
- Xie MZ, Li JL, Cai ZM, Li KZ, Hu BL: Impact of primary colorectal Cancer location on the KRAS status and its prognostic value. BMC Gastroenterol 2019, 19(1): 46.
- Cienfuegos JA, Baixauli J, Arredondo J, Pastor C, Martínez Ortega P, Zozaya G, Martí-Cruchaga P, Hernández Lizoáin JL: Clinico-pathological and oncological differences between right and left-sided colon cancer (stages I-III): analysis of 950 cases. Rev Esp Enferm Dig 2018, 110(3): 138-144.
Asia-Pacific Journal of Oncology
print ISSN: 2708-7980, online ISSN: 2708-7999
 Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript