Labeling of ethylenediamine tetramethylene phosphonate with 153Sm and 177Lu, comparison study
Hesham MH Zakaly1, 3, Mostafa Y. A. Mostafa1, 4, M Zhukovsky1, 2
1Ural Federal University, Yekaterinburg, Russia.
2Institute of Industrial ecology UB RAS, Ekaterinburg, Russia.
3Al-Azhar University, Assiut Branch, Physics Department, Assuit, Egypt.
4Minia University, Faculty of Science, Department of Physics, El-Minia, Egypt.
Correspondence: Hesham MH Zakaly (Ural Federal University, Yekaterinburg, Russia; E-mail: h.m.zakaly@azhar.edu.eg).
Asia-Pacific Journal of Oncology 2020, 1: 38-44. https://doi.org/10.32948/ajo.2020.10.03
Received: 23 Aug 2019 | Accepted: 01 Otc 2020 | Published online: 03 Otc 2020
Background 177Lu and 153Sm are perspective radionuclides in terms of applying to nuclear medicine. High-energy beta particles and the relative half-life of the radionuclide are used to achieve an effective palliative treatment of bone metastases.
Materials and methods The absorbed doses in different organs and tissues of 177Lu and 153Sm in ionic form and labeled with EDTMP are determined by IDAC-Dose 2.1 (Internal Dose Assessment by Computer) software and WinAct software which used to calculate cumulative activity. 177Lu and 153Sm are lanthanide radionuclide which actively accumulates in liver and bone when used in ionic form. In the case of labeling with EDTMP, the distribution and elimination of the drug occur according to the kinetics of a carrier, EDTMP. The using of osteotropic (Describing any drug etc. that is attracted to, and targets bone) complex allows creating a large dose in the pathological areas and minimizing damages in healthy organs and tissues.
Results The effective dose per administered activity is 0.189 mSv/MBq for 177Lu-ionic form, 0.232 mSv/MBq for 153Sm-ionic form and 0.242 mSv/MBq for 177Lu-EDTMP and 0.139 mSv/MBq for 153sm-EDTMP.
Conclusion 177Lu and 153Sm labeled with EDTMP are decreasing the liver dose absorption and increasing the bone surface absorption for more effective treatment and minimize side effects.
Key words 153Sm, 177Lu, EDTMP, IDAC-Dose 2.1, radiopharmaceuticals labeled
Radionuclide therapy (RNT), employing radiopharmaceuticals labeled with β ̄ conversion electron-emitting radionuclides, is effectively utilized for bone pain palliation, thus providing significant improvement in the quality of life of patients suffering from pain resulting from secondary skeletal metastases.The major challenge in developing effective agents for the palliative treatment of bone pain arising from skeletal metastasis is to ensure the delivery of an adequate dose of ionizing radiation at the site of the skeletal lesion with minimum radiation-induced bone marrow suppression. These in vivo features are governed by the tissue penetration range and, hence, on the energies of the β ̄ particles of the radionuclides used in the radiopharmaceutical preparations [1, 2].
Designing ideal radiopharmaceuticals for use as bone pain palliatives require the use of a moderate energy β ̄ emitter as a radionuclide and a suitable polyaminophosphonic acid as a carrier molecule. Owing to its suitable decay characteristics [T1/2 = 6.73 d, Eβ (max) = 497 keV, Eγ = 113 keV (6.4%), 208 keV (11%)] as well as the feasibility of large-scale production inadequate specific activity and radionuclidic purity using a moderate flux reactor, 177Lu could be considered as a promising radionuclide for palliative care in painful bone metastasis. The present study is, therefore, oriented toward the preparation of a comparison of 177Lu complex of ethylenediamine tetramethylene phosphonic acid (EDTMP) in various models, with a 153Sm-EDTMP been available for therapy1.
153Sm –is a radionuclide emitting beta radiation with an average energy of 0.223 MeV and accompanying gamma radiation with an energy of 103 Kev (yield 29 %). The half-life of 153Sm is 46.3 hours-which imposes territorial restrictions. That is, the production of radionuclide, preparation of the drug and therapy should be carried out in a very short time. In North America, the drug 153Sm-EDTMP (ethylene diamine tetramethylene phosphonate) has been available for therapy since 1997 [2]. Since the use of this drug is possible in any kind of cancer, which are accompanied by bone metastases,the potential market for radiopharmaceuticals is very large. 153Sm-oxabiphore is a drug used presently in Russia for the treatment of bone lesions – similar to foreign 153Sm-EDTMP.This complex is concentrated in the skeleton in proportion to osteoblastic activity. Pathological foci, where the accumulation is intense, can be visualized in studies in the gamma camera, that is, allows scintigraphy of a patient and monitor the treatment process. The drug is very quickly excreted from the blood. After intravenous 0.5-3 hours in the blood remains only 1 % of the drug. It is excreted in the urine almost completely after 6 hours [3-5]. The behavior of the whole drug in the body is due to the nature of the ligand distribution, and the radionuclide in the complex serves either for the treatment of the disease or for diagnosis. The distribution of the ligand EDTMP is identical to the distribution of other complexes, the isotropic to the bone tissue, for example, methylene diphosphonate (MDP) [2, 3, 6]. Nowadays, methylene diphosphonate labeled with radionuclide 99mTc is used for the diagnosis of bone anomalies. Due to the gamma radiation emitted by the 99mTc radionuclide, health care professionals can assess bone metabolism in detail and track where the drug accumulates most, making therapy being planned if necessary.
The purpose of this work is the assessment and study the effect of the drug carrier EDTMP (i.e. ethylene diamine tetramethylene phosphonate) on the ionic form of 177Lu and 153Sm . In addition, even in ionic form, the distribution of 177Lu is better than 153Sm more absorbed in the bone surface, red bone marrow, and kidney with low absorption in life.
The initial text block in the file is a comment block. For structuring the original design of the file into parts special words-separators are used. These words are the delimiters and are always typed in all uppercase letters. The activity distribution for 177Lu and 153Sm in ionic forms, 177Lu-EDTMP and 153Sm-EDTMP in different organs based on the model in Figure 1 are calculated as shown in Figure 2. The calculation is prepared for the diagnostic pathological area of bone tissue in a patient. The values of the transition factors of the substance from blood to organs are taken on the basis of a number of studies, converted from biokinetics for mice according to human anatomy [1, 8, 10-12] and are presented in Table 1. The input files of the WinAct software package are created on the basis of these results and compiled as shown in Figure 2.
From the output files, the leaveing rate of a particular drug from the blood is estimated. This is an important indicator for minimizing dose loads in the body as a whole. In addition, the percentage of activity retention for the preparations from the pathological nidus and organs play a major role in setting restrictions for the magnitude of the input activity of the drug.
The comparison of drugs based on radionuclide 177Lu with drugs that are currently used in medical practice for the treatment of bone metastases, such as 153Sm-EDTMP is the main task. As it is noted above, phosphonates form very stable compounds with radionuclides from a number of rare earth elements, that is, with 177Lu and 153Sm. Based on this, the calculation takes into account that the solution is not more than 1 % free radionuclide. Using the data mentioned above, the source files for 153Sm in combination with ethylene diamine tetramethylenePhosphonate.
When using a drug based on radionuclide and a therapeutic agent, the dynamics of behavior in a body completely depends on a carrier. That is, the calculation files for the drug 177Lu-EDTMP and 153Sm-EDTMP are identical, except for the half-life of the radionuclide. In addition, in the process of further calculation the presence in the solution of 1% free radionuclide will be taken into account, biokinetic data for which are not the same. A number of studies showed that the radiochemical resistance of drugs is not less than 99 %. Therefore, the free radionuclide 177Lu in each solution will be taken into account separately in the calculations.
WinAct generates three output files and an information file with an extension ".log", which basically duplicates the input data. All files received as a result of the program are located in the \output folder. The file extension ".act" contains information on the activity contained in an organ or tissue as a function of the time since the beginning of the nuclide intake. It is this file that was used to plot the dependence of the retention of activity on time after administration of the drug. The file extension ".ext" contains data on the rate of excretion of the nuclide with urine and feces (1/day) as a function of time. Additionally, the file tabulated data on the retention of the nuclide in the lungs and in the body as a function of time. In file with the extension ".u50" contains data on the number of nuclear transformations in a particular organ or tissue.
The output results from the WinAct program are used as input data for IDAC 2.1 software, an in-house dosimetry program for nuclear medicine based on the ICRP adult reference voxel phantoms [13, 14]. As a result, the absorbed doses to organs and tissues is estimated.
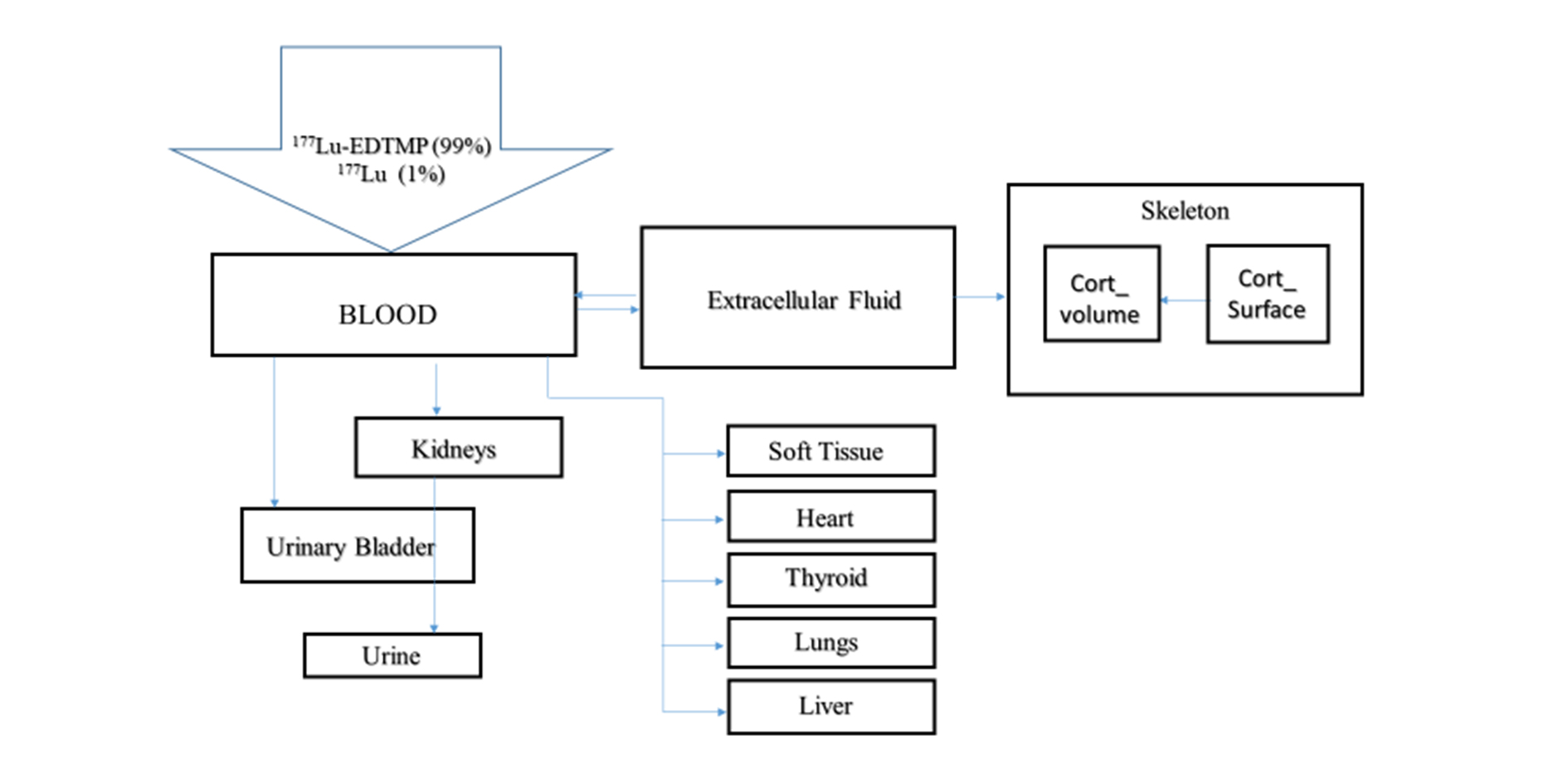 Figure 1. Biokinetic model of the drug 177Lu-EDTMP or 153Sm-EDTMP taking into account the conversion.
Figure 1. Biokinetic model of the drug 177Lu-EDTMP or 153Sm-EDTMP taking into account the conversion.
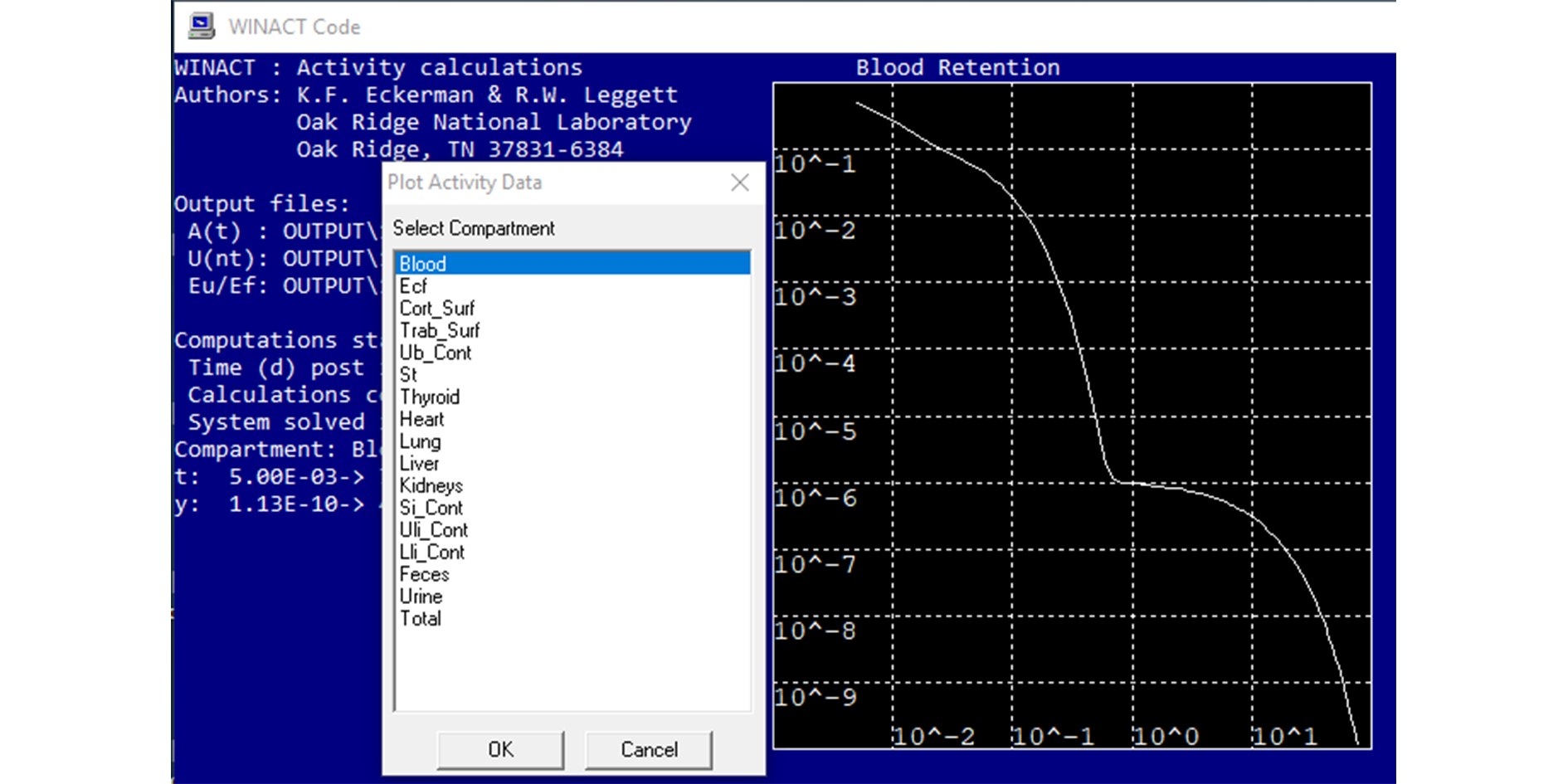 Figure 2. The input file of WinACt.
Figure 2. The input file of WinACt.
|
Table 1. Constant transition time between compartments of osteotropic drug EDTMP |
||
|
Path, |
Transition Rate K, Day-1 |
|
|
From |
To |
|
|
Blood |
->Ecf |
134 |
|
Ecf |
-> Blood |
20.79 |
|
Ecf |
->Cort_Boone_Surf |
10.39 |
|
Ecf |
->Trab_Boon_Surf |
10.39 |
|
Blood |
->UB_Cont |
16.03 |
|
Blood |
->Soft Tissue |
3.01 10-1 |
|
Blood |
-> Spleen |
5.20 10-2 |
|
Blood |
-> Heart |
1.10 10-2 |
|
Blood |
->Lungs |
3.84 10-2 |
|
Blood |
-> Liver |
9.04 10-2 |
|
Blood |
-> Kidneys |
1.12 10 1 |
|
Soft Tissue |
->Blood |
1.42 10-2 |
|
Spleen |
->Blood |
1.80 10-2 |
|
Heart |
->Blood |
7.95 10-2 |
|
Lungs |
->Blood |
2.21 10-2 |
|
Liver |
->Blood |
7.36 10-3 |
|
Kidneys |
->UB_Cont |
5.47 10-2 |
177Lu-EDTMP absorbed dose is two times more than 135Sm-EDTMP with nearly the same effects on other organs. In addition, 177Lu-EDTMP like 135Sm-EDTMP does not deposit in the liver unlike the ionic forms. The difference between 177Lu and 135Sm in their ionic forms seem larger than ``two times” in liver (Figure 5).
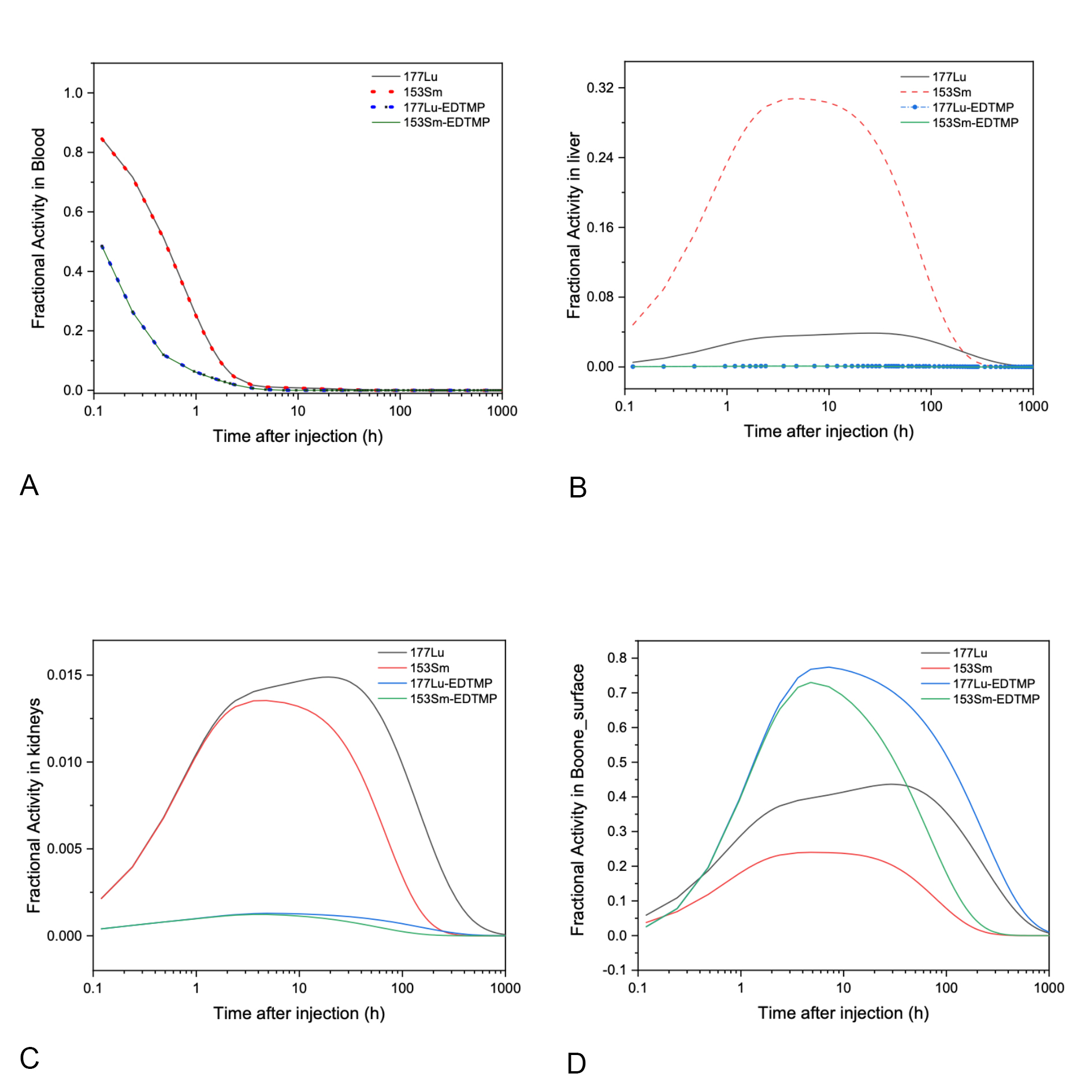 Figure 3. Fraction activities curves in blood and different organs for the three cases of interest.
Figure 3. Fraction activities curves in blood and different organs for the three cases of interest.
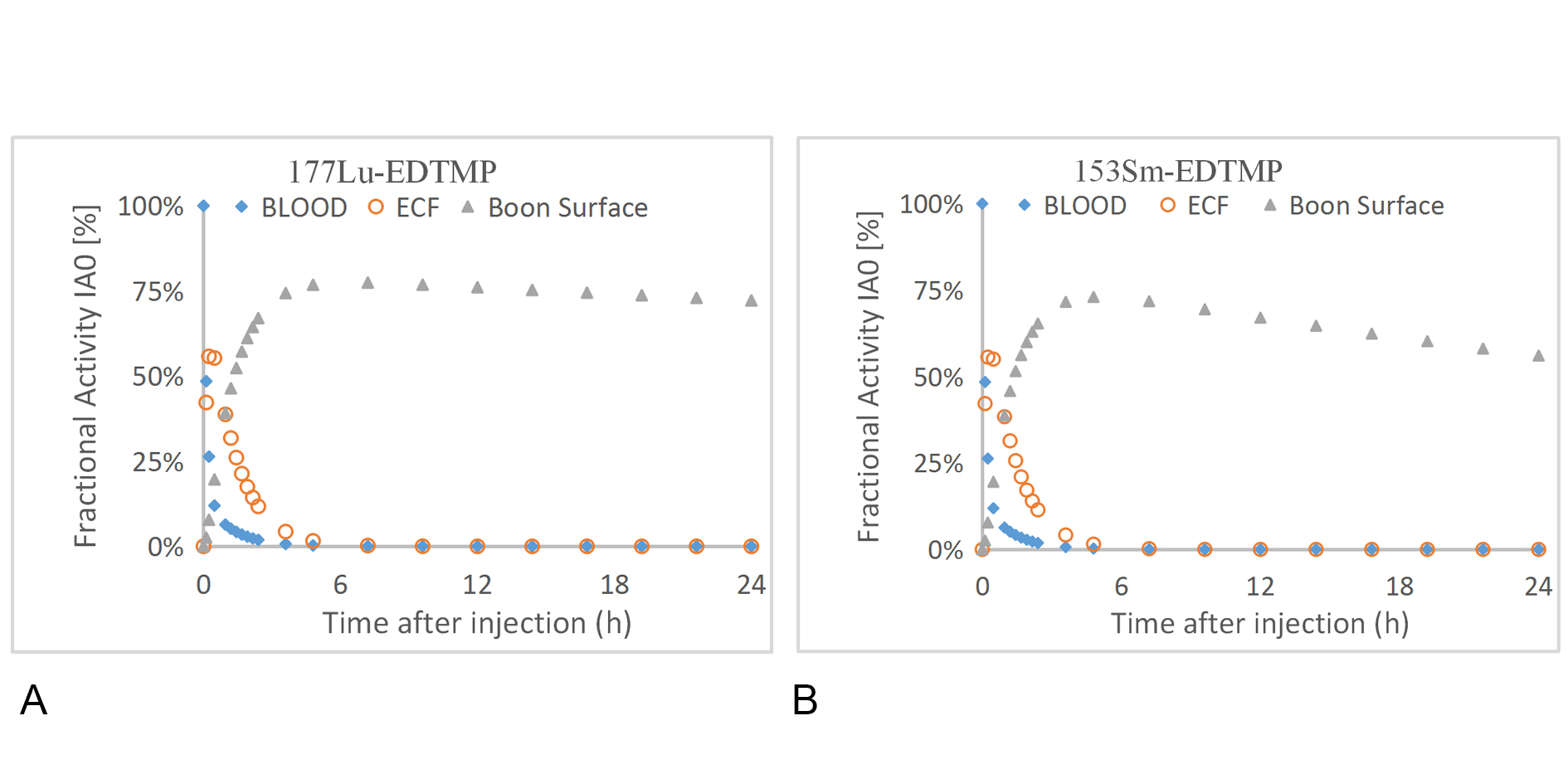 Figure 4. Distribution of activity in blood, ECF and bone surface of 177Lu-EDTMPand 153Sm-EDTMP.
Figure 4. Distribution of activity in blood, ECF and bone surface of 177Lu-EDTMPand 153Sm-EDTMP.
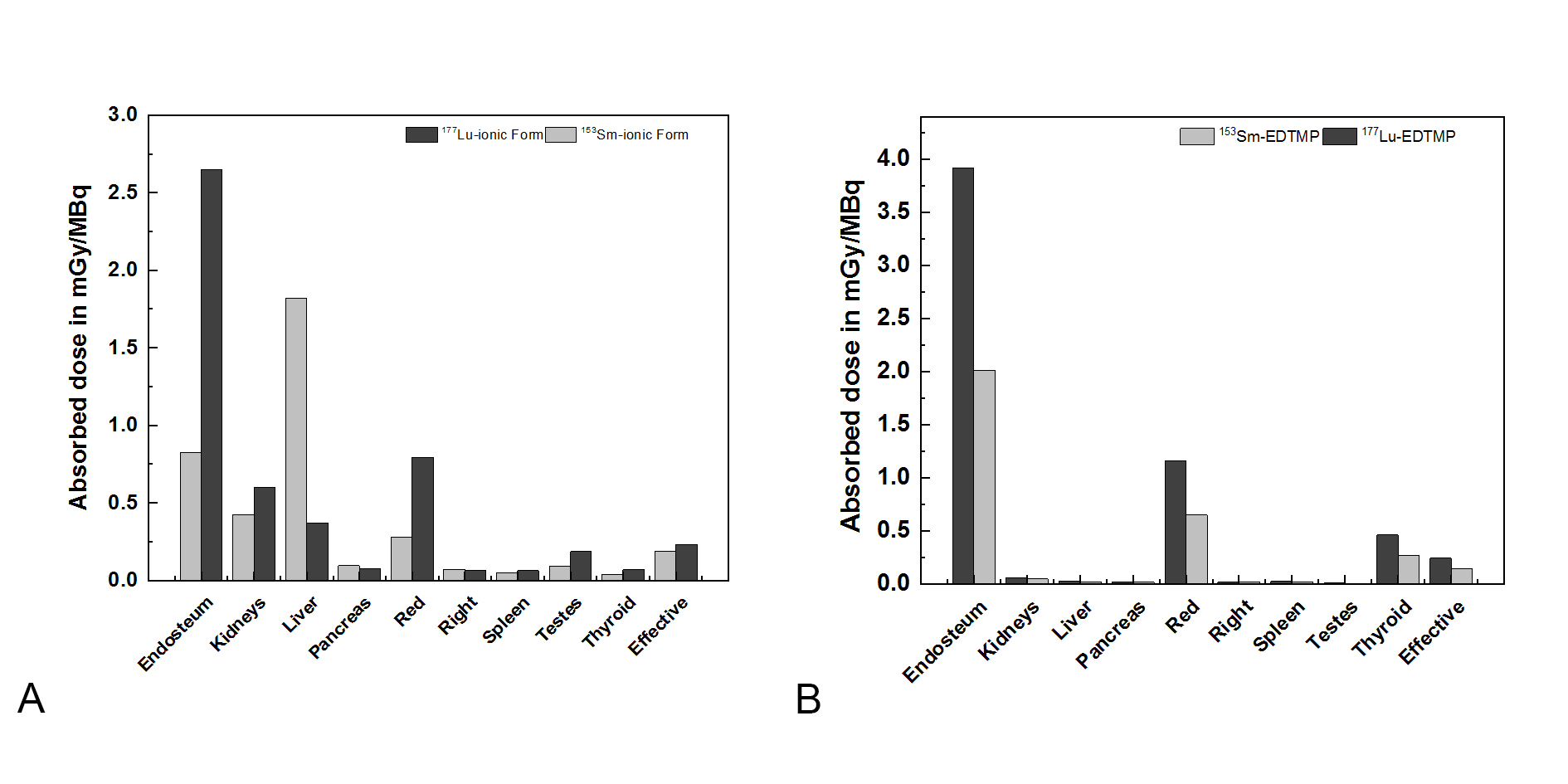 Figure 5. (A) 177Lu and 153SM ionic form absorbed Dose in (mSv/MBq) in different organs and tissues; (B)177Lu-EDTMP and 153Sm-EDTMP absorbed Dose in (mSv/MBq) in different organs and tissues.
Figure 5. (A) 177Lu and 153SM ionic form absorbed Dose in (mSv/MBq) in different organs and tissues; (B)177Lu-EDTMP and 153Sm-EDTMP absorbed Dose in (mSv/MBq) in different organs and tissues.
Figure 3 and 4 refer to the time-activity curves of the radiopharmaceutical as fitted by WinAct. The figures show that the behavior of fractional activity retention in different organs with 177Lu and 153Sm in Ionic form and when labeled with EDTMP. The pharmaceuticals in ionic form removed from blood slower than when labeled with EDTMP, this is appearing the advantages of using 177Lu-EDTMP instead of ionic form. The highest amount of activity cumulated in bone surface and little cumulated in other organs in case of 177Lu-EDTMP, this means that 177Lu-EDTMP is better than 135Sm-EDTMP in diagnostic of bone metastases disease. In addition, long time of removal from bone give us indicators for it is also better in therapeutic.
The peculiarity of this type of therapy is reasoned by the targeted action of the drug in the focus of the disease, a large dose is created and, very importantly, the dose load on healthy bone tissue, organs and tissues are minimized. The absorption in the bone tissue of a person with metastatic lesions of the skeleton depends on the degree of a disease, the general condition of a patient and many other factors. In the work, it is accepted that the absorption in the pathological area of bone tissue is 20% of the proportion of the substance deposited in a healthy skeleton. Figure 5 shows the results of comparing dose calculation conducted using two drugs.
177Lu-EDTMP absorbed dose is two times more than 135Sm-EDTMP with nearly the same effects on other organs. In addition, 177Lu-EDTMP like 135Sm-EDTMP does not deposit in the liver unlike the ionic forms. The difference between 177Lu and 135Sm in their ionic forms seem larger than ``two times” in liver.
The research involving experimentation on human or animal subjects was approved by the Ethics Committee in Ural Federal University, Yekaterinburg, Russia.
Author contributions
All authors contribute equally for writing this article.
Competing interests
None
- Chakraborty S, Das T, Banerjee S, Balogh L, Chaudhari PR, Sarma HD, Polyák A, Máthé D, Venkatesh M, Janoki G, Pillai MR: 177 Lu-EDTMP: A Viable Bone Pain Palliative in Skeletal Metastasis. Cancer Biother. Radiopharm 2008, 23(2): 202-213.
- Anderson P, Nuñez R: Samarium lexidronam (153Sm-EDTMP): Skeletal radiation for osteoblastic bone metastases and osteosarcoma. Expert Rev. Anticancer Ther 2007, 7(11): 1517-1527.
- Summary Of Product Characteristics: CIS bio international, member of IBA molecular group of companies. 2015.
- Finlay IG, Mason MD, Shelley M: Radioisotopes for the palliation of metastatic bone cancer: A systematic review. Lancet Oncol 2005, 6(6): 392-400.
- Pandit-Taskar N, Batraki M, Divgi CR: Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J Nucl Med 2004, 45(8): 1358-1365.
- Das T, Sarma HD, Shinto A, Kamaleshwaran KK, Banerjee S: Formulation, Preclinical Evaluation, and Preliminary Clinical Investigation of an In-House Freeze-Dried EDTMP Kit Suitable for the Preparation of 177 Lu-EDTMP. Cancer Biother Radiopharm 2014, 29(10): 412-421.
- Eckerman KF, Leggett RW, Williams LR: An Elementary Method for Solving Compartmental Models with Time-Dependent Coefficients. WinAct 2002.
- Zakaly HMH, Mostafa MYA, Zhukovsky M: Dosimetry Assessment of Injected 89Zr-Labeled Monoclonal Antibodies in Humans. Radiat Res 2019, 191(5): 466-474.
- Mostafa MYA, Zakaly HMH, Zhukovsky M: Assessment of exposure after injection of 99mTc-labeled intact monoclonal antibodies and their fragments into humans. Radiol Phys Technol 2019, 12(1): 96-104.
- Chakraborty S, Das T, Sarma HD, Venkatesh M, Banerjee S: Comparative studies of 177Lu-EDTMP and 177Lu-DOTMP as potential agents for palliative radiotherapy of bone metastasis. Appl Radiat Isot 2008, 66(9): 1196-1205.
- Vigna L, Matheoud R, Ridone S, Arginelli D, Della Monica P, Rudoni M, Inglese E, Brambilla M: Characterization of the [153Sm]Sm-EDTMP pharmacokinetics and Estimation of radiation absorbed dose on an individual Basis. Phys Medica 2011, 27(3): 144-152.
- Taylor DM, Leggett RW: A generic biokinetic model for predicting the behaviour of the lanthanide elements in the human body. Radiat Prot Dosimetry 2012, 105(1-4): 193-198.
- Andersson M, Johansson L, Eckerman K, Mattsson S: IDAC-Dose 2.1, an internal dosimetry program for diagnostic nuclear medicine based on the ICRP adult reference voxel phantoms. EJNMMI Res 2017, 7(1): 88.
- Liniecki J: Chapters 1-5. Ann ICRP 2011, 39: 15-49.
Asia-Pacific Journal of Oncology
print ISSN: 2708-7980, online ISSN: 2708-7999
 Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript