Research Article | Open Access
Characteristics and outcomes of patients with small cell lung cancer - findings from a New Zealand SACT database
Ross Lawrenson1, 2, Ha Nguyen1, Chunhuan Lao1, Rawiri Keenan1, Ian Kennedy2
1Medical Research Centre, University of Waikato, Hamilton, New Zealand.
2Te Whatu Ora Health New Zealand, Waikato, New Zealand.
Correspondence: Ross Lawrenson (Medical Research Centre, Te Huataki Waiora - School of Health, University of Waikato, Private Bag 3105, Hamilton 3240, New Zealand; Email: ross.lawrenson@waikatodhb.health.nz).
Asia-Pacific Journal of Oncology 2022, 4: 1-9. https://doi.org/10.32948/ajo.2023.02.02
Received: 16 Jan 2023 | Accepted: 02 Feb 2023 | Published online: 10 Feb 2023
Methods We used the custom-built SACT database collected by the Oncology Department at Waikato Hospital NZ, which recorded comprehensive lung cancer patient factors and SACT regimens from 2000 to 2021. We reported summary statistics to review the treatment by ethnicity, explored Kaplan Meier all-cause survival of patients, and estimated the unadjusted and adjusted odds ratios of surviving 12 months.
Results 742 patients with SCLC were included in this study, with 43% identified as Māori. Approximately 75% of patients received SACT, and there was no difference in the uptake of SACT between Māori and non-Māori. The median survival for SCLC was 8.5 months. After adjustment, patients treated with carboplatin plus etoposide (with/without durvalumab) or cisplatin plus etoposide were 1.5 times or 4.9 times respectively more likely to survive 12 months than those without a SACT regimen.
Conclusion There was no significant evidence of disparities in patterns of care and outcomes between Māori and non-Māori with SCLC. Carboplatin/cisplatin in combination with etoposide remained the primary first line SACT regimen for patients with SCLC in this New Zealand cancer treatment centre.
Keywords systemic anti-cancer treatment, small cell lung cancer, māori, equity
Systemic anti-cancer treatment (SACT) databases assist with audit and can be used to inform and improve the quality of SACT treatment. For instance, a SACT dataset has been developed as part of the National Cancer Registration and Analysis Service (NCRAS) within Public Health England [3]. In NZ, the Cancer Control Agency has begun the development of a national SACT system. Waikato Hospital is a regional cancer treatment centre that provides medical oncology treatment to a population of approximately 700,000 people, of whom 27% identify as Māori. The Oncology Department at Waikato Hospital has used a custom-built SACT database and chemotherapy prescribing system since 2000. The system records patient characteristics and their systemic chemotherapy regimens and is linked to the national mortality database to record patient outcomes.
SCLC is an aggressive subtype of lung cancer associated with smoking, with a high propensity to metastasis and a poor prognosis [4]. In our region, SCLC accounts for 16% of pathologically reported lung cancer cases. In Māori diagnosed with lung cancer, the proportion is higher at 23% [5]. Most patients with SCLC have advanced disease at diagnosis. Patients are usually treated with combination treatment consisting of either intravenous carboplatin plus etoposide, or intravenous cisplatin plus etoposide. Concurrent radiation therapy with etoposide and cisplatin is the standard of care for patients with limited-stage disease in whom cure may be possible [6]. The addition of immunotherapy agents such as durvalumab [7] or atezolizumab [8] offers benefit over conventional chemotherapy. However, these agents are currently not publicly funded in NZ for this indication. Complete remission is uncommon [9]. Most patients develop progressive disease after first line chemotherapy and generally derive only modest benefit from subsequent lines of treatment. The median survival from diagnosis for SCLC is reported to be as low as seven months [10]. Current NZ guidelines suggest patients with advanced lung cancer without significant co-morbidities should be offered SACT. The New Zealand Lung Cancer Standards include recommendations on the use of SACT in both patients with SCLC and non-small cell lung cancer (NSCLC) [2]. Currently in NZ 71.3% of patients with SCLC are estimated to receive SACT. There is variability between District Health Boards (DHBs) but the number of people treated in many individual DHBs is small [2].
This study aimed to document the characteristics and outcomes of SCLC patients referred to the Waikato Hospital Oncology Department over a 20-year period.
This is a retrospective study based on data prospectively collected from a purpose-built SACT chemotherapy prescribing and oncology database system. Patients are those with a first diagnosis of SCLC referred to the Medical Oncology Unit based at Waikato Hospital. The SACT system was first used in 2000, and all patients recorded from then until 31 December 2021 have been included in this study.
Data collected on patients include their age, gender, year of diagnosis, ethnicity (Māori or non-Māori), BMI, date of cancer diagnosis, cancer cell type (small cell or non-small cell), cancer stage, SACT regimens prescribed and date of death. Cancer stage was initially collected as limited or extensive, but more recently TNM staging has been used. Those with T4 or M1 metastatic disease were categorised as “extensive” while all others were categorised as “limited” stage.
Analysis
We first reviewed the treatment by ethnicity of patients with SCLC to see if there were differences in the proportion of patients who received a first SACT regimen versus the proportion of patients without SACT. We then looked at the proportion of Māori versus non-Māori who received a second treatment regimen. We calculated the all-cause survival of patients from diagnosis with no intravenous SACT (IV SACT) regimen, carboplatin plus etoposide, cisplatin plus etoposide or other. We also looked at the survival of those patients receiving second or subsequent lines of therapy. We calculated survival in those receiving a SACT regimen by ethnicity, BMI category and year of diagnosis using the Kaplan Meier method.
Finally, we estimated the unadjusted and adjusted odds ratios of surviving 12 months by age, gender, ethnicity, year of diagnosis, cancer stage (limited/I/II/III and extensive/IV), and regimens (no IV SACT, carboplatin + etoposide with/without durvalumab, cisplatin + etoposide, and other). We also report the unadjusted and adjusted odds ratios of surviving 12 months in those receiving carboplatin and etoposide as their first-line treatment.
Results
Flow chart of the SACT database (2000 - 2021) (Figure 1).
Univariate analysis
We identified 742 cases of SCLC in the departmental database with SCLC. Of the SCLC patients, 43% identified as Māori. Overall, 560/742 (75.5%) of patients received a primary course of SACT. There was no difference in the uptake of SACT between Māori and non-Māori, although Māori were average five years younger and were more likely to be female. Māori receiving SACT were twice as likely to have a BMI of 30 or above (Table 1).
Of patients who had SACT, 454/560 (81%) of patients were initially treated with carboplatin and etoposide. Of these patients, 18% (83/454) received a second line of SACT, which was mainly cyclophosphamide adriamycin vincristine (CAV) or paclitaxel (96%). Among the 175 patients with the limited-stage disease, 45 were initially treated with cisplatin and etoposide.
Overall median survival was 8.5 months. Thirty-five percent of patients receiving SACT survived 12 months, while for those who did not start SACT, only 23% survived 12 months. The median survival in those receiving SACT increased from 7 months between 2000 and 2004 to 9 months from 2015 to 2021.
Multivariate analysis
Table 2 showed that the key factors associated with improved 12-month survival included younger age, year of diagnosis, limited stage of disease rather than extensive and the use of SACT regimens (carboplatin + etoposide with/without durvalumab and cisplatin + etoposide). Importantly Māori versus non-Māori ethnicity was not associated with survival (adjusted odds ratio: 1.19; 95% CI: 0.82 - 1.71). After adjustment, patients treated with carboplatin and etoposide (with/without durvalumab) or cisplatin and etoposide were 1.5 times and 4.9 times respectively more likely to survive 12 months than those without a SACT regimen. Table 3 shows that patients with the limited-stage of disease receiving carboplatin and etoposide as their first-line therapy were 2.6 times more likely to survive 12 months than those with extensive stage.
Kaplan Meier survival analysis
The Kaplan Meier curves (Figure 2) confirm the expected overall poor prognosis with a 5-year survival of less than 10%. Consistent with published data, patients with limited-stage disease receiving cisplatin and etoposide had a 5-year survival substantially better at close to 30%. For those patients who receive carboplatin based SACT (Figure 3), a proportion received a second SACT regimen. This group have an improved median survival by 2.5 years. It is not possible to differentiate if this improved survival is due to the use of the second regimen, or simply a reflection of survivorship bias. Despite improved median survival, beyond two years from diagnosis there was no difference in the percentage of patients alive based on the number of regimens received. By the Kaplan-Meier method, there is no difference in survival for Māori compared to non-Māori (Figure 4). An increased BMI is associated with improved survival in those receiving SACT (Figure 5). The 1-year survival was less than 30% during 2000-2004, and the survival rate increased to 40% during 2015-2021 (Figure 6).
|
Table 1. Summary statistics. |
|||||||||||
|
Patients received the first SACT regimen (560) |
Patients received the second SACT regimen (105) |
Patients received no IV SACT regimen (182) |
All patients |
||||||||
|
Factors |
Non-Māori |
Māori |
p-value |
Non-Māori |
Māori |
p-value |
Non-Māori |
Māori |
p-value |
Total |
|
|
N=317 |
N=243 |
N=59 |
N=46 |
N=103 |
N=79 |
N=742 |
|||||
|
Age group |
<50 |
11 (3.5%) |
21 (8.6%) |
<0.01 |
4 (6.8%) |
9 (19.6%) |
0.09 |
7 (6.8%) |
6 (7.6%) |
0.17 |
45 (6.1%) |
|
50-54 |
18 (5.7%) |
33 (13.6%) |
5 (8.5%) |
7 (15.2%) |
5 (4.9%) |
10 (12.7%) |
66 (8.9%) |
||||
|
55-59 |
27 (8.5%) |
51 (21.0%) |
8 (13.6%) |
11 (23.9%) |
8 (7.8%) |
11 (13.9%) |
97 (13.1%) |
||||
|
60-64 |
54 (17.0%) |
48 (19.8%) |
10 (16.9%) |
8 (17.4%) |
14 (13.6%) |
13 (16.5%) |
129 (17.4%) |
||||
|
65-69 |
83 (26.2%) |
37 (15.2%) |
14 (23.7%) |
5 (10.9%) |
18 (17.5%) |
9 (11.4%) |
147 (19.8%) |
||||
|
70-74 |
68 (21.5%) |
34 (14.0%) |
12 (20.3%) |
5 (10.9%) |
22 (21.4%) |
16 (20.3%) |
140 (18.9%) |
||||
|
75-79 |
37 (11.7%) |
15 (6.2%) |
4 (6.8%) |
1 (2.2%) |
14 (13.6%) |
10 (12.7%) |
76 (10.2%) |
||||
|
≥80 |
19 (6.0%) |
4 (1.6%) |
2 (3.4%) |
0 (0.0%) |
15 (14.6%) |
4 (5.1%) |
42 (5.7%) |
||||
|
Age (years) (mean ± SD) |
|
67 (9) |
62 (9) |
<0.01 |
64 (9) |
58 (9) |
<0.01 |
68 (11) |
64 (10) |
0.01 |
65 (10) |
|
Gender |
Male |
156 (49.2%) |
92 (37.9%) |
<0.01 |
27 (45.8%) |
16 (34.8%) |
0.26 |
55 (53.4%) |
31 (39.2%) |
0.06 |
334 (45.0%) |
|
Female |
161 (50.8%) |
151 (62.1%) |
32 (54.2%) |
30 (65.2%) |
48 (46.6%) |
48 (60.8%) |
408 (55.0%) |
||||
|
BMI |
<18.5 |
20 (6.3%) |
10 (4.1%) |
<0.01 |
1 (1.7%) |
0 (0.0%) |
0.02 |
1 (1.0%) |
0 (0.0%) |
0.44 |
31 (4.2%) |
|
18.5-24.9 |
135 (42.6%) |
61 (25.1%) |
21 (35.6%) |
7 (15.2%) |
4 (3.9%) |
1 (1.3%) |
201 (27.1%) |
||||
|
25-29.9 |
101 (31.9%) |
71 (29.2%) |
20 (33.9%) |
13 (28.3%) |
2 (1.9%) |
1 (1.3%) |
175 (23.6%) |
||||
|
≥30 |
61 (19.2%) |
99 (40.7%) |
17 (28.8%) |
26 (56.5%) |
2 (1.9%) |
0 (0.0%) |
162 (21.8%) |
||||
|
Unknown |
0 (0.0%) |
2 (0.8%) |
0 (0.0%) |
0 (0.0%) |
94 (91.3%) |
77 (97.5%) |
173 (23.3%) |
||||
|
Cancer stage |
Limited/I/II/III |
77 (24.3%) |
60 (24.7%) |
0.91 |
16 (27.1%) |
12 (26.1%) |
0.73 |
21 (20.4%) |
17 (21.5%) |
0.75 |
175 (23.6%) |
|
Extensive/IV |
226 (71.3%) |
174 (71.6%) |
40 (67.8%) |
33 (71.7%) |
79 (76.7%) |
61 (77.2%) |
540 (72.8%) |
||||
|
Unstaged |
14 (4.4%) |
9 (3.7%) |
3 (5.1%) |
1 (2.2%) |
3 (2.9%) |
1 (1.3%) |
27 (3.6%) |
||||
|
Regimen |
Carboplatin + etoposide |
261 (82.3%) |
193 (79.4%) |
0.94 |
7 (11.9%) |
5 (10.9%) |
0.96 |
- |
|||
|
Cisplatin + etoposide |
28 (8.8%) |
27 (11.1%) |
0 (0.0%) |
0 (0.0%) |
|||||||
|
Carboplatin |
12 (3.8%) |
11 (4.5%) |
2 (3.4%) |
1 (2.2%) |
|||||||
|
CAV |
10 (3.2%) |
8 (3.3%) |
36 (61.0%) |
30 (65.2%) |
|||||||
|
Paclitaxel |
1 (0.3%) |
1 (0.4%) |
14 (23.7%) |
10 (21.7%) |
|||||||
|
Carboplatin + etoposide + durvalumab |
5 (1.6%) |
3 (1.2%) |
0 (0.0%) |
0 (0.0%) |
|||||||
|
12-month survival |
No |
208 (65.6%) |
151 (62.1%) |
0.40 |
21 (35.6%) |
14 (30.4%) |
0.58 |
80 (77.7%) |
60 (75.9%) |
0.78 |
499 (67.3%) |
|
Yes |
109 (34.4%) |
92 (37.9%) |
38 (64.4%) |
32 (69.6%) |
23 (22.3%) |
19 (24.1%) |
243 (32.7%) |
||||
|
Median survival (8.5 months) |
No |
140 (44.2%) |
111 (45.7%) |
0.72 |
7 (11.9%) |
8 (17.4%) |
0.42 |
71 (68.9%) |
51 (64.6%) |
0.53 |
373 (50.3%) |
|
Yes |
177 (55.8%) |
132 (54.3%) |
52 (88.1%) |
38 (82.6%) |
32 (31.1%) |
28 (35.4%) |
369 (49.7%) |
||||
|
Year |
2000-2004 |
69 (21.8%) |
38 (15.6%) |
0.02 |
4 (6.8%) |
6 (13.0%) |
0.15 |
36 (35.0%) |
11 (13.9%) |
<0.01 |
154 (20.8%) |
|
2005-2009 |
83 (26.2%) |
48 (19.8%) |
19 (32.2%) |
9 (19.6%) |
22 (21.4%) |
16 (20.3%) |
169 (22.8%) |
||||
|
2010-2014 |
81 (25.6%) |
67 (27.6%) |
15 (25.4%) |
19 (41.3%) |
18 (17.5%) |
22 (27.8%) |
188 (25.3%) |
||||
|
2015-2021 |
84 (26.5%) |
90 (37.0%) |
21 (35.6%) |
12 (26.1%) |
27 (26.2%) |
30 (38.0%) |
231 (31.1%) |
||||
|
Table 2. Odds ratio of surviving 12 months. |
||||
|
Variables |
Unadjusted OR |
95% CI |
Adjusted OR |
95% CI |
|
Age (years) |
0.955*** |
0.939 - 0.971 |
0.955*** |
0.937 - 0.974 |
|
Gender |
|
|
|
|
|
Male |
1 |
- |
1 |
- |
|
Female |
1.460* |
1.062 - 2.005 |
1.324 |
0.931 - 1.884 |
|
Ethnicity |
|
|
|
|
|
Māori |
1 |
- |
1 |
- |
|
Non-Māori |
0.857 |
0.626 - 1.173 |
1.186 |
0.824 - 1.706 |
|
Year of diagnosis |
|
|
|
|
|
2000-2004 |
1 |
- |
1 |
- |
|
2005-2009 |
1.349 |
0.810 - 2.247 |
1.387 |
0.794 - 2.422 |
|
2010-2014 |
2.160** |
1.341 - 3.479 |
2.235** |
1.323 - 3.778 |
|
2015-2021 |
1.653* |
1.040 - 2.628 |
1.647 |
0.985 - 2.754 |
|
Cancer stage |
|
|
|
|
|
Extensive/IV |
1 |
- |
1 |
- |
|
Limited/I/II/III |
4.233*** |
2.958 - 6.058 |
3.294*** |
2.202 - 4.927 |
|
Regimen |
|
|
|
|
|
No IV SACT regimen |
1 |
- |
1 |
- |
|
Carboplatin + etoposide (with/without durvalumab) |
1.538* |
1.032 - 2.293 |
1.539* |
1.005 - 2.356 |
|
Cisplatin + etoposide |
11.333*** |
5.468 - 23.492 |
4.853*** |
2.208 - 10.664 |
|
Other |
0.971 |
0.427 - 2.209 |
1.085 |
0.461 - 2.557 |
|
Note: *** p<0.001, ** p<0.01, * p<0.05. Odds ratio (OR). Confidence interval (CI). |
||||
|
Table 3. Odds ratio of surviving 12 months in the group receiving carboplatin and etoposide as their first line treatment. |
||||
|
Variables |
Unadjusted OR |
95% CI |
Adjusted OR |
95% CI |
|
Age (years) |
0.967** |
0.946 - 0.989 |
0.961** |
0.938 - 0.985 |
|
Gender |
|
|
|
|
|
Male |
1 |
. |
1 |
. |
|
Female |
1.469 |
0.976 - 2.211 |
1.306 |
0.849 - 2.008 |
|
Ethnicity |
|
|
|
|
|
Māori |
1 |
. |
1 |
. |
|
Non-Māori |
0.962 |
0.642 - 1.440 |
1.351 |
0.847 - 2.154 |
|
Year of diagnosis |
|
|
|
|
|
2000-2004 |
1 |
. |
1 |
. |
|
2005-2009 |
1.502 |
0.795 - 2.837 |
1.532 |
0.781 - 3.002 |
|
2010-2014 |
1.536 |
0.836 - 2.820 |
1.890 |
0.994 - 3.594 |
|
2015-2021 |
1.510 |
0.838 - 2.722 |
1.808 |
0.968 - 3.378 |
|
Cancer stage |
|
|
|
|
|
Extensive/IV |
1 |
. |
1 |
. |
|
Limited/I/II/III |
2.444*** |
1.509 - 3.956 |
2.565*** |
1.544 - 4.262 |
|
BMI |
|
|
|
|
|
<18.5 |
1 |
. |
1 |
. |
|
18.5-24.9 |
2.048 |
0.661 - 6.348 |
2.397 |
0.741 - 7.760 |
|
25-29.9 |
2.274 |
0.731 - 7.076 |
2.632 |
0.809 - 8.560 |
|
≥30 |
2.801 |
0.898 - 8.742 |
3.080 |
0.942 - 10.075 |
|
Note: *** p<0.001, ** p<0.01, * p<0.05. Odds ratio (OR). Confidence interval (CI). |
||||
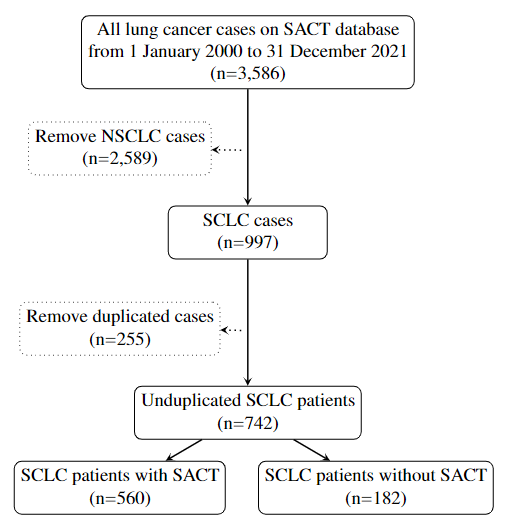 Figure 1. Flow chart of the SACT database (2000 - 2021).
Figure 1. Flow chart of the SACT database (2000 - 2021).
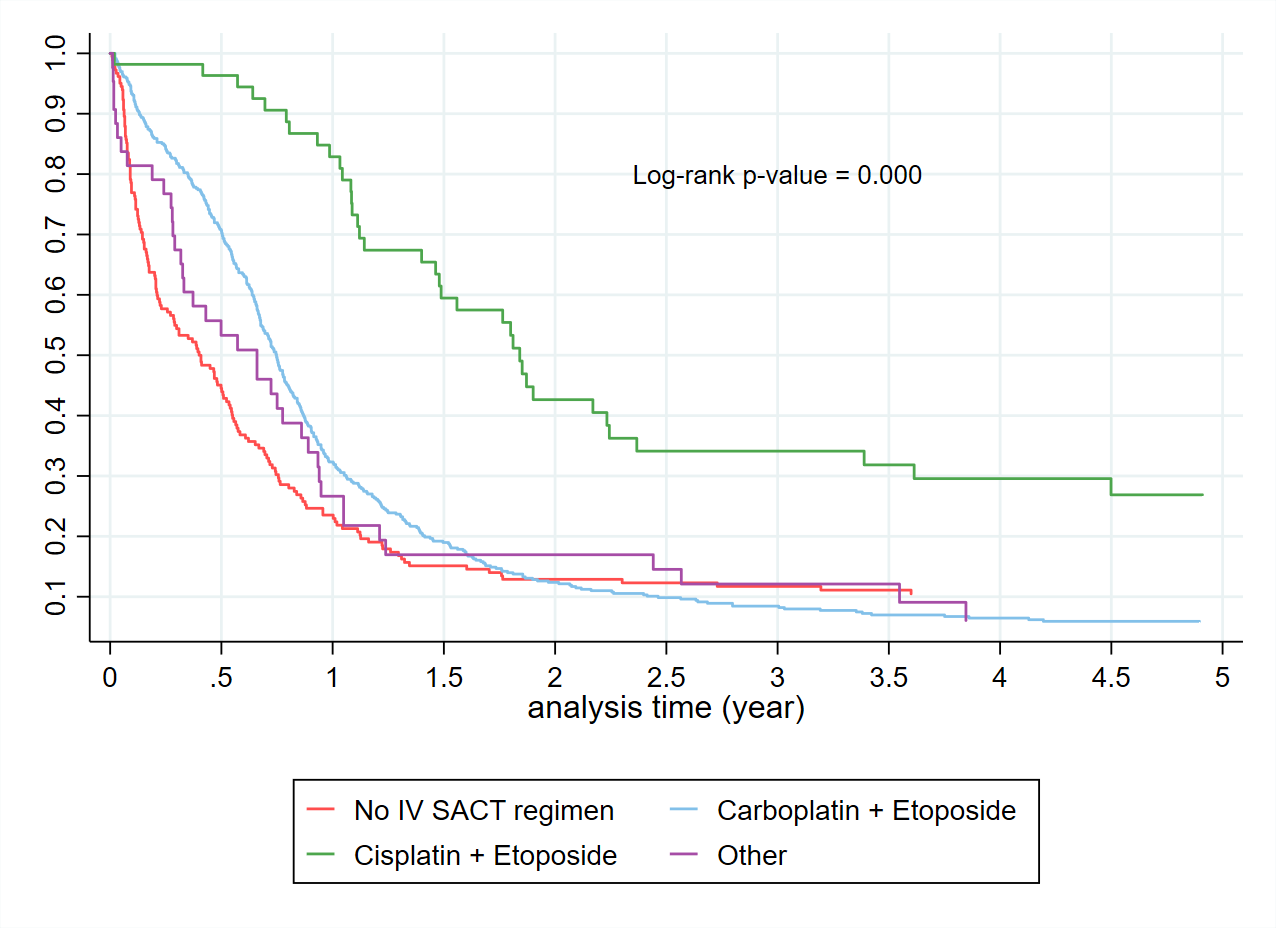 Figure 2. Kaplan Meier curves of patients with the first line intravenous treatment vs without.
Figure 2. Kaplan Meier curves of patients with the first line intravenous treatment vs without.
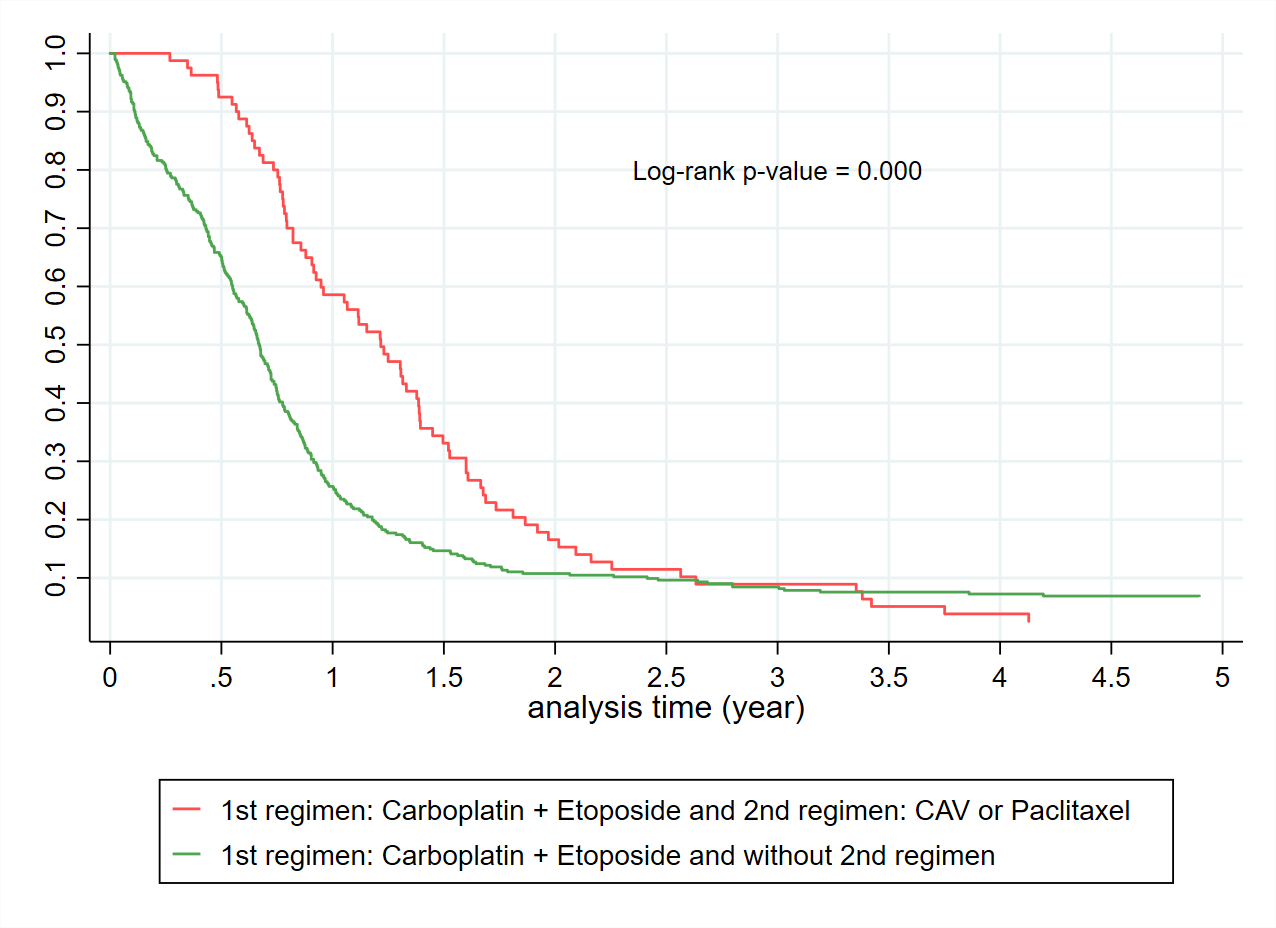 Figure 3. Kaplan Meier curves of patients who were originally treated with carboplatin + etoposide and then had the second regimen CAV/Paclitaxel vs without the second regimen.
Figure 3. Kaplan Meier curves of patients who were originally treated with carboplatin + etoposide and then had the second regimen CAV/Paclitaxel vs without the second regimen.
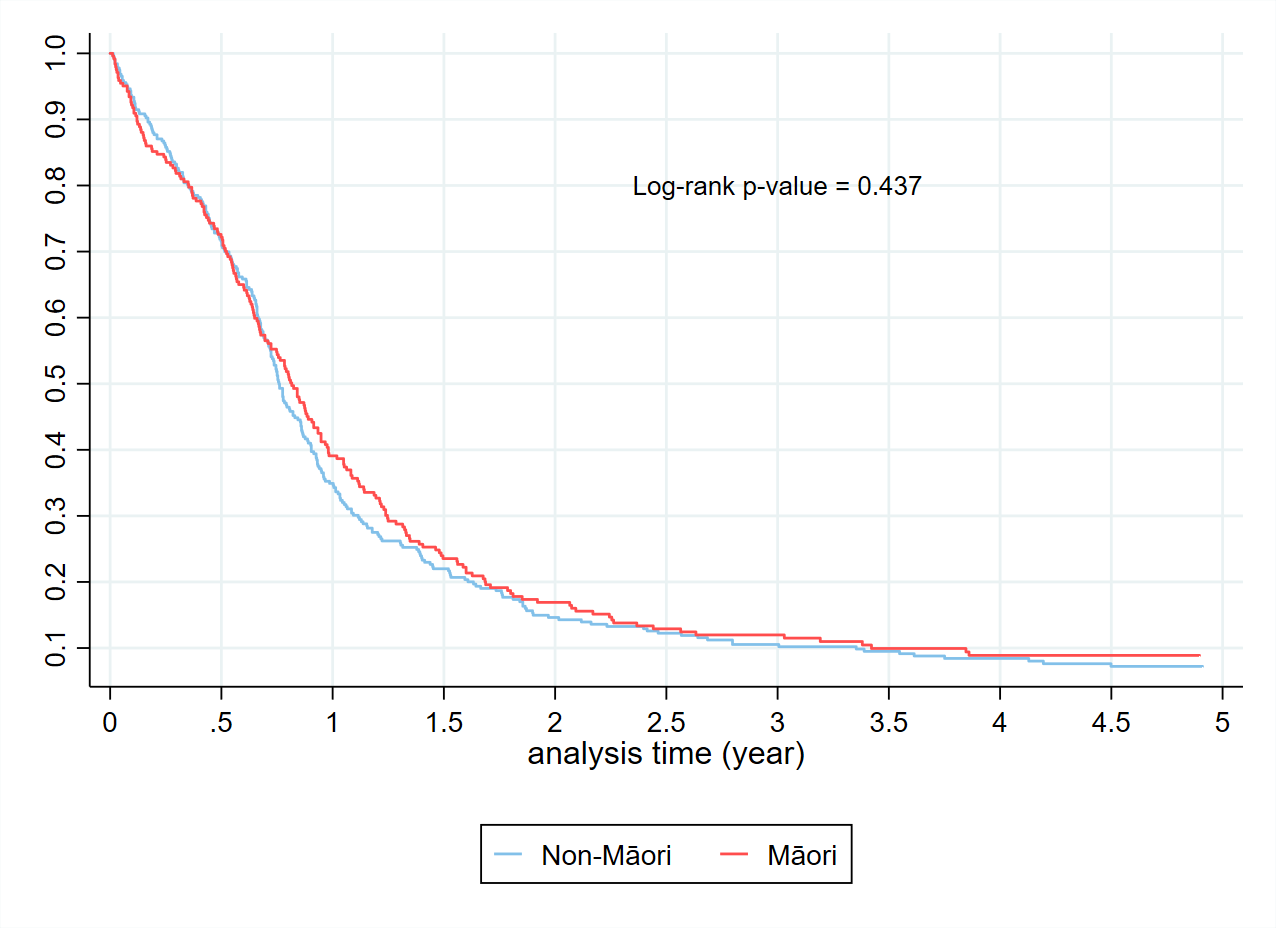 Figure 4. Kaplan Meier curves in those receiving a SACT regimen by ethnicity.
Figure 4. Kaplan Meier curves in those receiving a SACT regimen by ethnicity.
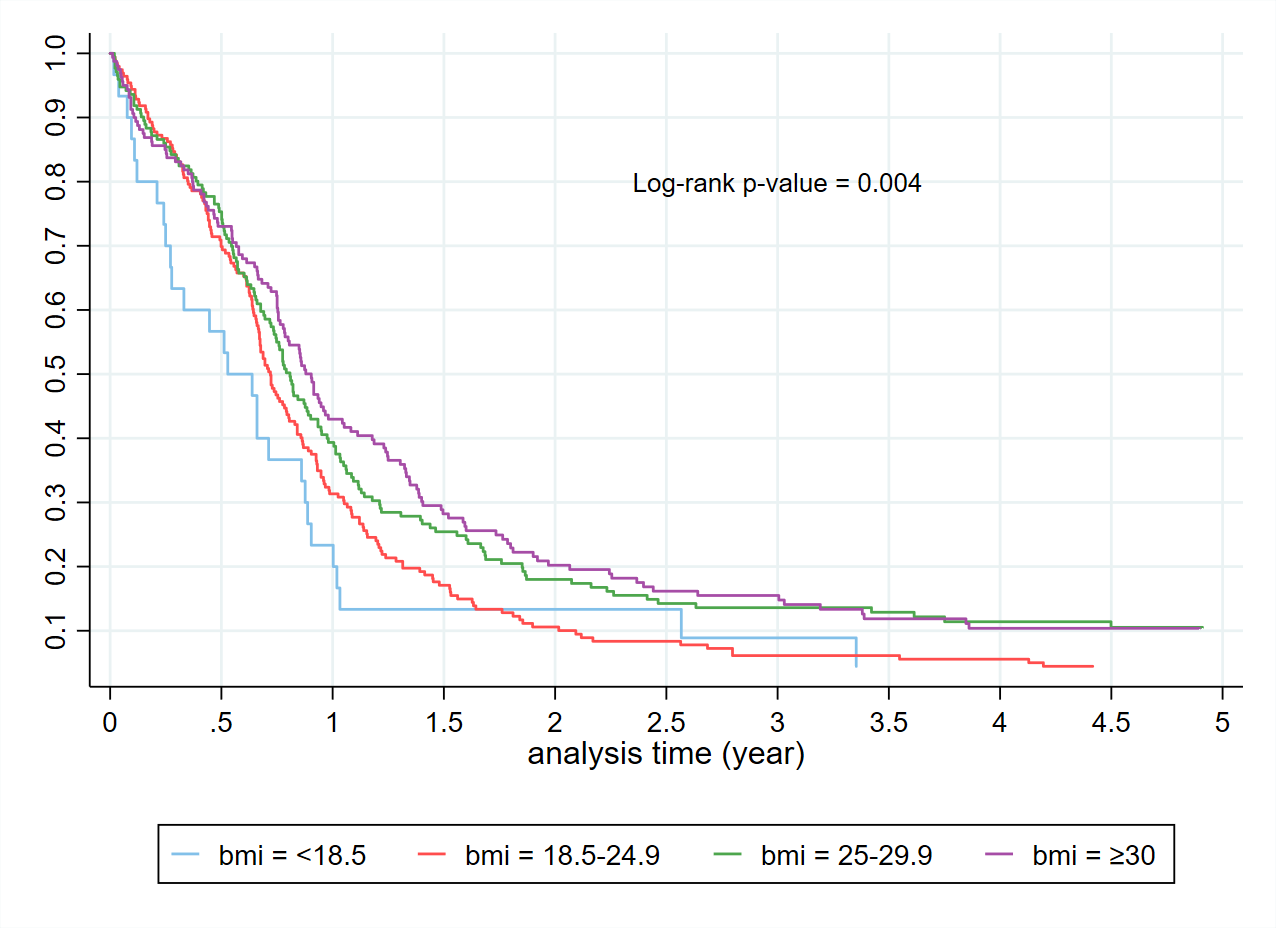 Figure 5. Kaplan Meier curves in those receiving a SACT regimen by BMI.
Figure 5. Kaplan Meier curves in those receiving a SACT regimen by BMI.
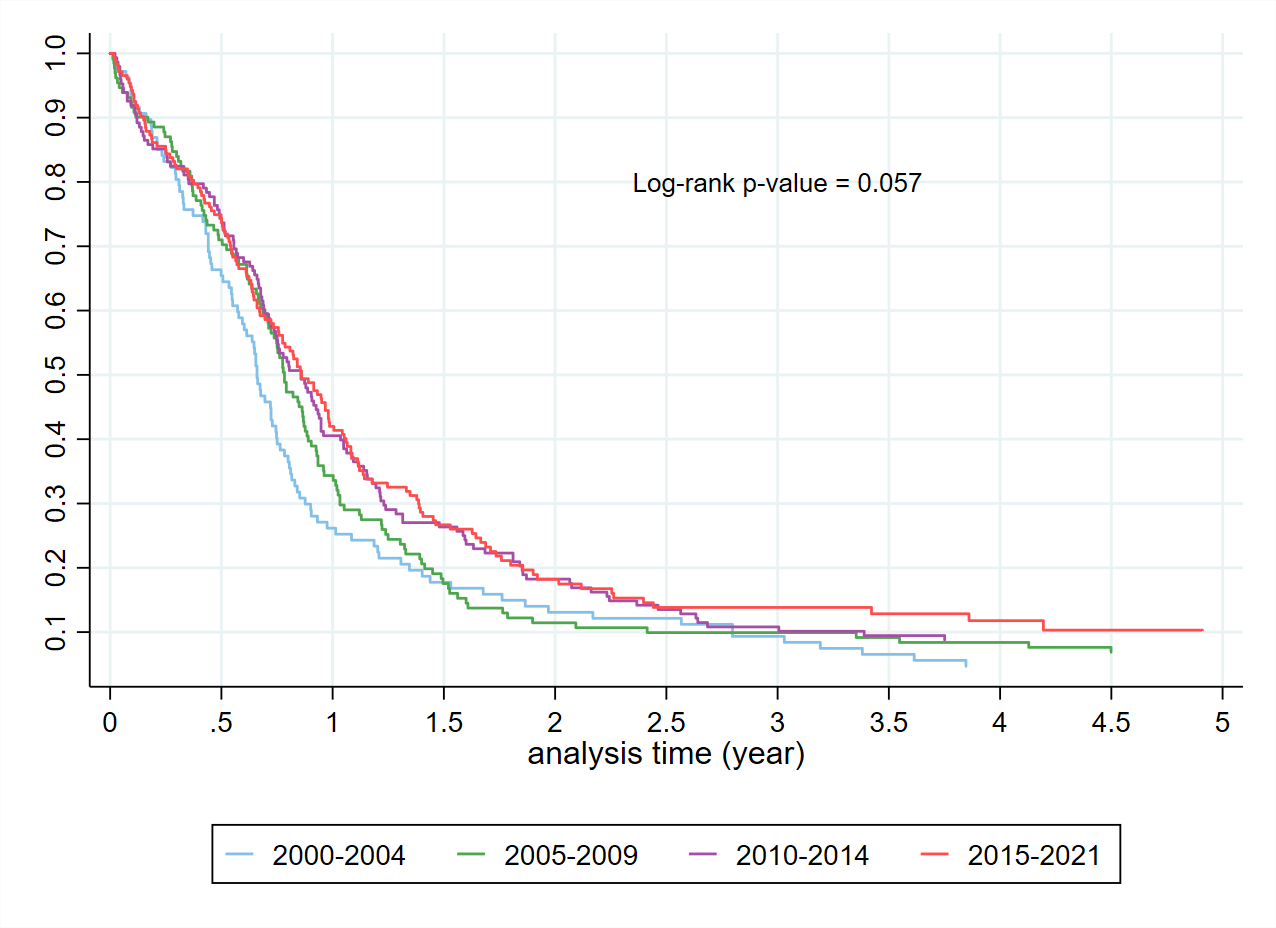 Figure 6. Kaplan Meier curves in those receiving a SACT regimen by year of diagnosis.
Figure 6. Kaplan Meier curves in those receiving a SACT regimen by year of diagnosis.
In our study cohort, only 18% of patients treated with the first line SACT also received the second line therapy primarily CAV or paclitaxel. Previous research found that the use of CAV as the second line chemotherapy produced a response rate of 13% to 28% [20-22] .There was evidence that the single-agent paclitaxel was moderately effective in patients with previously treated SCLC. The use of the single-agent paclitaxel in those patients showed a response rate of 24% to 29% [23]. The second line treatment may improve overall survival in lung cancer patients or provide significant palliation of symptoms [23]. However, the value of the second line therapy is insignificant in patients with chemo-resistant or refractory disease [24].
New treatments are needed to prolong survival for patients diagnosed with SCLC. Only very few patients were fortunate enough to receive international standard of care immunotherapy as part of a named patient compassionate access scheme. In 2019, the standard treatment for extensive-stage SCLC evolved when the addition of atezolizumab, a programmed death ligand 1 (PD-L1) - targeted immune checkpoint inhibitor (ICI), to carboplatin plus etoposide was shown to improve survival over chemotherapy alone [8, 25, 26]. The median overall survival in those receiving atezolizumab was 12.3 months [8]. The CASPIAN trial in 2019 showed that patients with extensive-stage SCLC receiving durvalumab (an anti-PD-L1 ICI) in combination with carboplatin/cisplatin plus etoposide also had a median survival of around 13 months [7, 26]. The overall two-year survival of patients treated with durvalumab plus chemotherapy was 22%, which was higher than that of patients with chemotherapy only (14% [7]. A phase III KEYNOTE-604 study in 2020 reported that the addition of pembrolizumab (anti-PD-1) to the standard first line therapy etoposide plus platinum significantly improved progression-free survival [27], but insignificantly increased overall survival in patients with extensive-stage SCLC [28]. According to current guidelines of NCCN, thoracic consolidation radiation therapy (TCRT) is recommended for extensive stage SCLC patients with response to systemic chemotherapy. However, the selection of patients and radiotherapy doses are not well defined [29].
Survival by the five-year diagnosis cohort improved modestly over the 20-year period of the study. Our reported survival for limited-stage patients receiving combined modality treatment is comparable to published literature [30]. A prior study reported that the median survival of limited-stage and extensive-stage SCLC was only 14-20 months and 8-13 months respectively [12]. Importantly, we detected no systematic difference in terms of the pattern of care or survival outcomes based on ethnicity. Survival of Māori and non-Māori with SCLC was similar with the hazard ratio at 12 months being 1.20 (95% CI: 0.84 - 1.73). In our SACT database, approximately 75.5% of Māori with SCLC received SACT during 2000-2021. Lung Cancer Quality Improvement Monitoring Report of Cancer Control Agency NZ reported 75.3% of Māori with SCLC treated with SACT based on the National Cancer Register during 2015-2018 [2]. Lung Cancer Quality Performance Indicator Report of Cancer Control Agency NZ emphasised the importance of timely and fair access to SACT for Māori diagnosed with lung cancer to improve their survival and quality of life [31].
A strength of this study is that the departmental database provides comprehensive information on lung cancer patients receiving SACT, including age, gender, ethnicity, BMI, cancer stage, SACT regimens and survival over 20 years.
One of the limitations is that the current SACT database does not include information on co-morbidities, which may affect the survival probabilities of lung cancer patients. Māori are more likely to have co-morbidities than non-Māori at diagnosis. In addition, non-Māori is a mixed group including a small proportion of Asian and Pacific patients, who may have different SACT regimens and outcomes.
We would like to acknowledge the support from the Oncology Department at Waikato Hospital New Zealand in providing the SACT dataset.
Ethical policy
The study was approved by the Health and Disability Ethics Committee - Ministry of Health, New Zealand (Ref. No. 2022 AM 5900).
Author contributions
Ross Lawrenson designed the study. Ross Lawrenson, Ha Nguyen, Chunhuan Lao and Ian Kennedy wrote the paper and analysed data. Rawiri Keenan edited the paper and ensured a Māori equity lens was applied to the interpretation of the findings.
Competing interests
All authors disclose no competing interests.
Funding
This study was funded by the Waikato Medical Research Foundation (#336) and Health Research Council of New Zealand (HRC Ref ID: 21/990).
- Cancer patient survival: 1994 to 2011. In: Ministry of Health. Epub ahead of print.
- Cancer Control Agency. Lung Cancer Quality Improvement Monitoring Report. Epub ahead of print.
- NCRAS. SACT Chemotherapy Dataset. In. Epub ahead of print.
- Rudin CM, Brambilla E, Faivre-Finn C, Sage J: Small-cell lung cancer. Nat Rev Dis Primers 2021, 7(1): 3.
- Lawrenson R, Lao C, Brown L, Wong J, Middleton K, Firth M, Aitken D: Characteristics of lung cancers and accuracy and completeness of registration in the New Zealand Cancer Registry. N Z Med J 2018, 131(1479): 13-23.
- Noronha V, Sekhar A, Patil VM, Menon N, Joshi A, Kapoor A, Prabhash K: Systemic therapy for limited stage small cell lung carcinoma. J Thorac Dis 2020, 12(10): 6275-6290.
- Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, et al: Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394(10212): 1929-1939.
- Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, et al: First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018, 379(23): 2220-2229.
- Konala VM, Madhira BR, Ashraf S, Graziano S: Use of Immunotherapy in Extensive-Stage Small Cell Lung Cancer. Oncology 2020, 98(11): 749-754.
- Wang S, Tang J, Sun T, Zheng X, Li J, Sun H, Zhou X, Zhou C, Zhang H, Cheng Z, et al: Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep 2017, 7(1): 1339.
- Kalemkerian GP, Akerley W, Bogner P, Borghaei H, Chow LQ, Downey RJ, Gandhi L, Ganti AK, Govindan R, Grecula JC, et al: Small cell lung cancer. J Natl Compr Canc Netw 2013, 11(1): 78-98.
- Demedts IK, Vermaelen KY, van Meerbeeck JP: Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J 2010, 35(1): 202-215.
- Farago AF, Keane FK: Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res 2018, 7(1): 69-79.
- Pass HI, Carbone DP, Johnson DH, Minna JD, Scagliotti GV, Turrisi AT: Principles and Practice of Lung Cancer: the official reference text of the International Association for the Study of Lung Cancer (IASLC) Epub ahead of print.: Lippincott Williams & Wilkins; 2012.
- Kalemkerian GP: Running in Place: The 20th Anniversary of the NCCN Small Cell Lung Cancer Guidelines Panel. J Natl Compr Canc Netw 2015, 13(6): 704-706.
- Karam I, Jiang SY, Khaira M, Lee CW, Schellenberg D: Outcomes of small cell lung cancer patients treated with cisplatin-etoposide versus carboplatin-etoposide. Am J Clin Oncol 2015, 38(1): 51-54.
- Azar I, Yazdanpanah O, Jang H, Austin A, Kim S, Chi J, Alkassis S, Saha BK, Chopra A, Neu K, et al: Comparison of Carboplatin With Cisplatin in Small Cell Lung Cancer in US Veterans. JAMA Netw Open 2022, 5(10): e2237699.
- Dingemans AC, Früh M, Ardizzoni A, Besse B, Faivre-Finn C, Hendriks LE, Lantuejoul S, Peters S, Reguart N, Rudin CM, et al: Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2021, 32(7): 839-853.
- Skarlos DV, Samantas E, Kosmidis P, Fountzilas G, Angelidou M, Palamidas P, Mylonakis N, Provata A, Papadakis E, Klouvas G, et al: Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group study. Ann Oncol 1994, 5(7): 601-607.
- Gervais R, Le Caer H, Monnet I, Falchero L, Baize N, Olivero G, Thomas P, Berard H, Auliac JB, Chouaid C: Second-line oral chemotherapy (lomustine, cyclophosphamide, etoposide) versus intravenous therapy (cyclophosphamide, doxorubicin, and vincristine) in patients with relapsed small cell lung cancer: a randomized phase II study of GFPC 0501. Clin Lung Cancer 2015, 16(2): 100-105.
- Shepherd FA, Evans WK, MacCormick R, Feld R, Yau JC: Cyclophosphamide, doxorubicin, and vincristine in etoposide- and cisplatin-resistant small cell lung cancer. Cancer Treat Rep 1987, 71(10): 941-944.
- Sculier JP, Klastersky J, Libert P, Ravez P, Brohee D, Vandermoten G, Michel J, Thiriaux J, Bureau G, Schmerber J, et al: Cyclophosphamide, doxorubicin and vincristine with amphotericin B in sonicated liposomes as salvage therapy for small cell lung cancer. Eur J Cancer 1990, 26(8): 919-921.
- Yamamoto N, Tsurutani J, Yoshimura N, Asai G, Moriyama A, Nakagawa K, Kudoh S, Takada M, Minato Y, Fukuoka M: Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res 2006, 26(1b): 777-781.
- Froeschl S, Nicholas G, Gallant V, Laurie SA: Outcomes of second-line chemotherapy in patients with relapsed extensive small cell lung cancer. J Thorac Oncol 2008, 3(2): 163-169.
- Calles A, Aguado G, Sandoval C, Álvarez R: The role of immunotherapy in small cell lung cancer. Clin Transl Oncol 2019, 21(8): 961-976.
- Iams WT, Porter J, Horn L: Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol 2020, 17(5): 300-312.
- Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, Cheema PK, Rodriguez-Abreu D, Wollner M, Yang JC, et al: Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol 2020, 38(21): 2369-2379.
- Reddy HG, Qin A, Kalemkerian GP: Emerging drugs for small cell lung cancer: a focused review on immune checkpoint inhibitors. Expert Opin Emerg Drugs 2020, 25(3): 353-366.
- Mitin T, Jain A, Degnin C, Chen Y, Henderson M, Thomas CR, Jr: Current patterns of care for patients with extensive stage small cell lung cancer: Survey of US radiation oncologists on their recommendations regarding thoracic consolidation radiotherapy. Lung Cancer 2016, 100: 85-89.
- Turrisi AT, 3rd, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, Wagner H, Aisner S, Johnson DH: Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999, 340(4): 265-271.
- Cancer Control Agency. Lung Cancer Quality Performance Indicators: Descriptions. Epub ahead of print.
Asia-Pacific Journal of Oncology
print ISSN: 2708-7980, online ISSN: 2708-7999
 Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript