Review Article | Open Access
Navigating in the labyrinth of thrombotic and bleeding risks in patients with malignancies – how to make the most reasonable choices for personalized anticoagulation?
Katarzyna (Kate) Rygiel1
1Department of Family Practice, Medical University of Silesia (SUM), Zabrze, Poland.
Correspondence: Katarzyna (Kate) Rygiel (Department of Family Practice, Medical University of Silesia (SUM), 3 Maja St, 41-800 Zabrze, Poland ; E-mail: kasiaalpha@yahoo.co.uk).
Asia-Pacific Journal of Oncology 2020, 1: 68-76. https://doi.org/10.32948/ajo.2020.12.31
Received: 23 Dec 2020 | Accepted: 31 Dec 2020 | Published online: 31 Dec 2020
Venous thromboembolism (VTE) frequently occurs among patients with malignancies and poses an important cause of morbidity and mortality in this population. Therefore, effective and safe thromboprophylaxis for oncology patients at the increased risk of VTE is of utmost importance. Commonly used anticancer treatments, including hormonal therapy (HT), chemotherapy (CHT), targeted therapy (TT), immune therapy (IT), radiotherapy (RT), and anti-angiogenesis monoclonal antibodies, as well as surgical procedures have been associated with VTE. For this reason, risk stratification scores, including tumor site, laboratory parameters, and patient’s clinical characteristics can help most accurately identify those patients, who will take the greatest advantage of a personalized approach to VTE.
This mini-review discusses cancer-related VTE risk stratification scores (e.g., the Khorana, Vienna Cancer and Thrombosis (CATS), and PROTECHT) that have been very useful for the detection of patients at the highest risk of VTE, who require an individual choice of the anticoagulant. This article briefly summarizes the updated American Society of Clinical Oncology (ASCO) clinical guidelines for the prevention and treatment of VTE in patients with cancer. In particular, it presents the direct oral anticoagulants (DOACs) as a new opportunity for both the preventive and therapeutic approach to VTE in this population. Furthermore, this overview provides some practical implications of the ASCO recommendations to the decision-making regarding safe and effective, personalized anticoagulant selection in various clinical setting. Hopefully, blending the patient’s medical context and personal preferences into VTE risk stratification scores will contribute to progress in the management of cancer-related VTE.
Key words Venous thromboembolism (VTE), non-vitamin K antagonist oral anticoagulants (NOACs), direct oral anticoagulants (DOACs), VTE risk stratification scores, Khorana Score (KS), cancer-related VTE
Cancer-related hypercoagulability includes type I (caused by the enzymatic degradation of endogenous heparin by heparanase from a tumor) and type II (relevant to factors linked to the patient, the tumor, and its therapy) [3]. Moreover, patients with malignancies, who are anticoagulated, have a higher incidence of bleeding compared with patients without cancer, regardless of the selected anticoagulation agent [4]. In addition, the patients, who suffer from metastatic disease, gastrointestinal (GI), gynecological or genitourinary (GU) cancers, thrombocytopenia, coagulopathy, and major bleeding episode, are usually characterized by higher bleeding risk [4].
Under these circumstances, clinical cancer-related VTE risk stratification scores have been useful to detect oncology patients with the greatest risk of VTE (Figure 1) [5-7]. Such risk scores include the primary anatomic site and histologic type of cancer, complete blood count (CBC) before administration of chemotherapy (CHT) (e.g., hemoglobin level, white blood cells, and platelets), body mass index (BMI), and soluble biomarkers (e.g., D-dimer and P-selectin) (Figure 1) [5-7].
This mini-review discusses the role of cancer-related VTE risk stratification scores (e.g., the Khorana, Vienna Cancer and Thrombosis (CATS), and PROTECHT) that have been very helpful (according to the results of the main randomized controlled trials (RCTs) focused on cancer-related VTE) for the identification of patients with the highest risk of VTE, who require an individual choice of the most optimal anticoagulant. Furthermore, it briefly summarizes the updated American Society of Clinical Oncology (ASCO) clinical guidelines for the management of VTE among patients with cancer. In particular, it presents the direct oral anticoagulants (DOACs), such as apixaban, edoxaban, and rivaroxaban, as a new treatment opportunity for both the prevention and treatment of VTE in patients with cancer. This overview also provides some practical implications of the ASCO recommendations to the decision-making regarding safe and effective, individualized anticoagulant choices in the oncology practice setting. Hopefully, blending the patient clinical context and personal preferences into VTE risk stratification scores will contribute to some progress in therapy and prevention of cancer-related VTE.
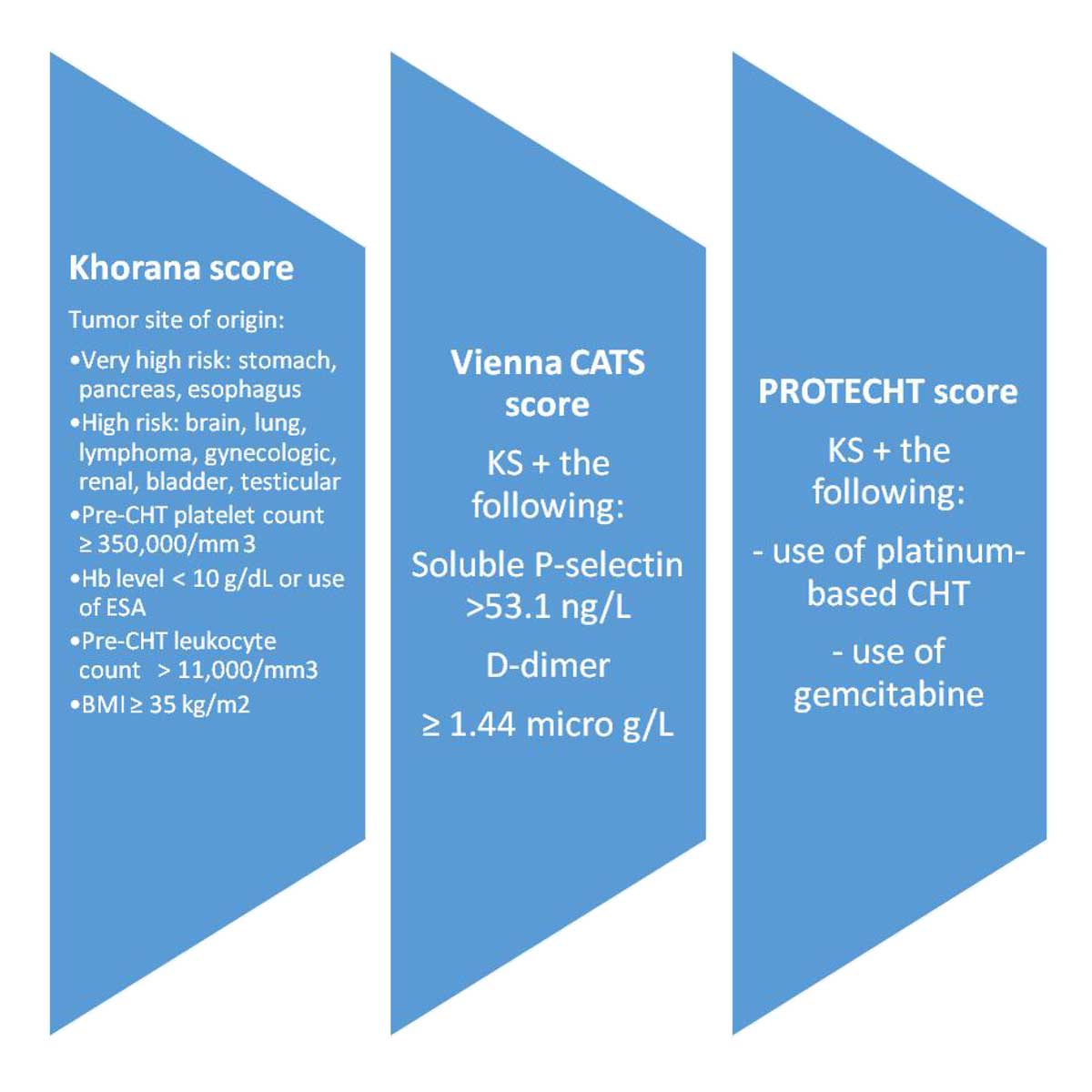 Figure 1. Risk stratification scores (Khorana, Vienna Cancer and Thrombosis (CATS), PROTECHT) for VTE risk assessment in patients with cancer [5, 6, 7]. Abbreviations: BMI, body mass index; CHT, chemotherapy; ESA, erythropoietin stimulating agent; Hb, hemoglobin; VTE, venous thromboembolism.
Figure 1. Risk stratification scores (Khorana, Vienna Cancer and Thrombosis (CATS), PROTECHT) for VTE risk assessment in patients with cancer [5, 6, 7]. Abbreviations: BMI, body mass index; CHT, chemotherapy; ESA, erythropoietin stimulating agent; Hb, hemoglobin; VTE, venous thromboembolism.
In general, a hypercoagulable state among patients with cancers has been categorized as type I (which occurs when the proportion between the endogenous heparin production and its degradation is altered, via accelerated degradation of endogenous heparin by an enzyme heparanase, secreted by a tumor), and type II (which involves other factors relevant to the malignant tumor itself, its treatment, and to the patient clinical or personal profile) [3]. Type I cancer-related hypercoagulability is characterized by recurrent VTE episodes, in patients with malignancies, due to an insufficient amount of the endogenous heparin, to keep the blood in its natural liquid state. This is predominantly caused by a degradation of the endogenous heparin by heparanase (an enzyme endoglycosidase), secreted by a tumor [10]. In fact, pancreatic cancers are characterized by the heparanase mRNA levels, which are above 30-times higher, compared to the ones encountered physiologically in the pancreatic gland [11]. Moreover, it has been shown that overexpression of heparanase was associated with poor prognosis in a majority of patients with lung cancer [12]. Similarly, a large meta-analysis of patients with gastric cancer has revealed that higher heparanase expression in gastric cancer was related to the tumor invasiveness, metastases to lymph nodes, and TNM stage [13]. Furthermore, some tumors can contribute to the narrowing of blood vessel’s lumen and slowing down local circulation, which in turn generates abnormal hemodynamic forces leading to endothelial dysfunction that often results in VTE [14]. In addition, the tissue factor (TF) represents a common signal for coagulation, and in particular, the TF upregulation in some cancers may lead to a hypercoagulable state [15]. Similarly, the upregulation of lysyl oxidase (LOX), which is an enzyme responsible for the cross-linking of collagen, and for the increase of platelet’s reactivity, can also elevate the VTE risk in many patients with neoplastic diseases [16]. Overexpression of heparanase and its excessive secretion by cancer cells often leads to a degradation of endogenous heparin and increased coagulability. In addition, heparanase can induce TF expression in vascular endothelium and neoplastic cells [17]. Type II cancer-related hypercoagulability predominantly relates to VTE events, which are not connected with the decreased levels of endogenous heparin. Type II predominantly involves a combination of stasis (secondary to pressure exerted on blood vessels by a tumor mass), the patient’s poor performance status, obesity, and anti-neoplastic therapy-related thrombosis (Figure 2) [18]. In addition, numerous anticancer therapies, such as hormonal therapy (HT), chemotherapy (CHT), targeted therapy (TT), immune therapy (IT), radiotherapy (RT), and anti-angiogenesis monoclonal antibodies are related to an elevated risk of VTE. Moreover, many patients who undergo surgery, often associated with the insertion of central venous catheters (or other devices), are immobilized or inactive, and thus, have a higher risk of VTE. (Figure 2) [18].
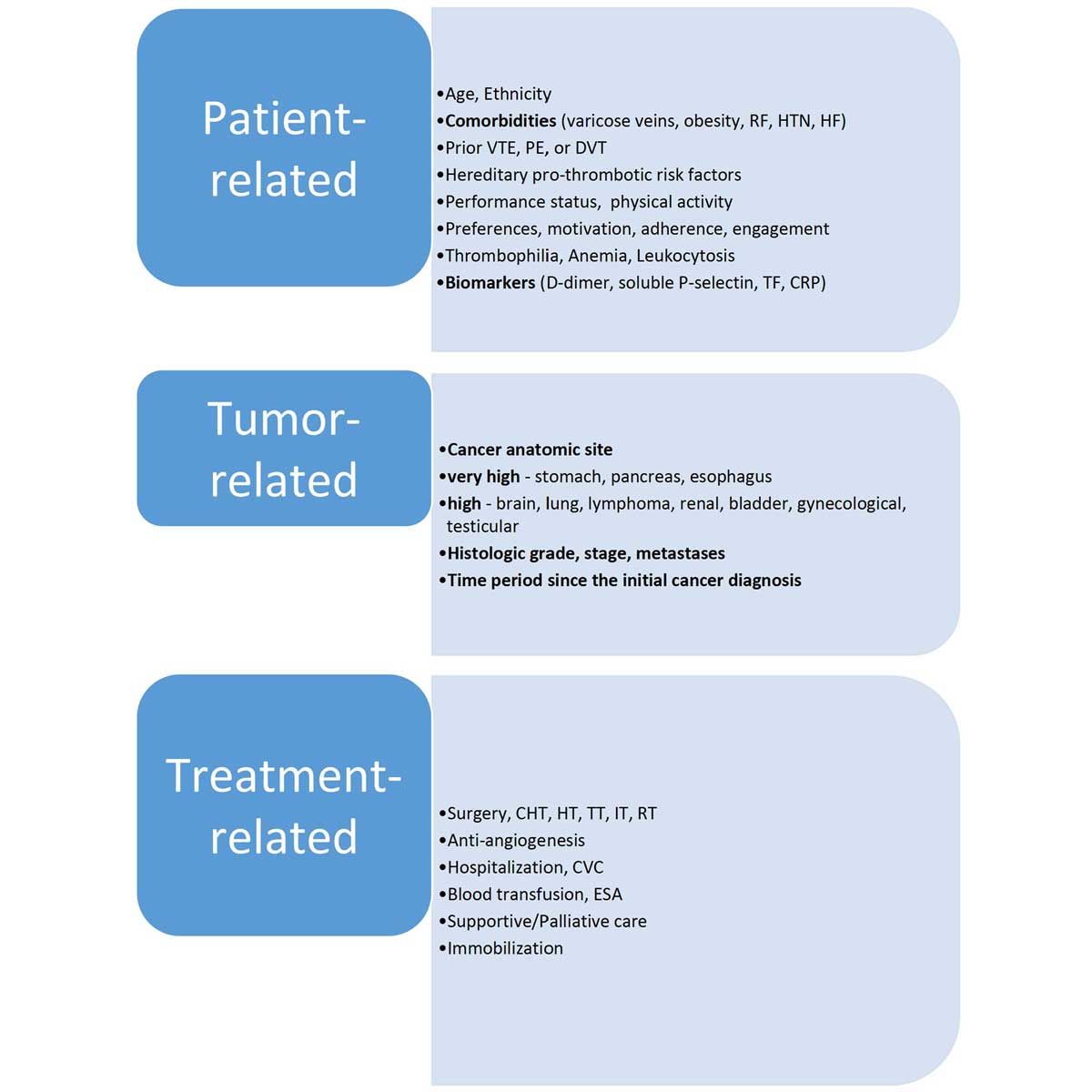 Figure 2. The main risk factors for thromboembolism in patients with cancer [18]. Abbreviations: CRP, C-reactive protein; DVT, deep venous thrombosis; ESA, erythropoietin stimulating agent; HF, heart failure; HTN, arterial hypertension; PE, pulmonary embolism; TF, tissue factor; VTE, venous thromboembolism; CHT, Chemotherapy; HT, Hormonal therapy; TT, Targeted therapy; IT, Immunotherapy; RT, Radiotherapy; CVC, central venous catheters; RF, renal failure; Hereditary pro-thrombotic risk factors (e.g., protein C deficiency, protein S deficiency, anti-thrombin deficiency, or factor V Leiden).
Figure 2. The main risk factors for thromboembolism in patients with cancer [18]. Abbreviations: CRP, C-reactive protein; DVT, deep venous thrombosis; ESA, erythropoietin stimulating agent; HF, heart failure; HTN, arterial hypertension; PE, pulmonary embolism; TF, tissue factor; VTE, venous thromboembolism; CHT, Chemotherapy; HT, Hormonal therapy; TT, Targeted therapy; IT, Immunotherapy; RT, Radiotherapy; CVC, central venous catheters; RF, renal failure; Hereditary pro-thrombotic risk factors (e.g., protein C deficiency, protein S deficiency, anti-thrombin deficiency, or factor V Leiden).
Numerous clinical prediction scores have been created to best identify those patients who may need primary VTE prophylaxis, due to their individual risk profiles (Figure 1) [5]. The Khorana score was the first, well-validated clinical tool to predict VTE risk, specifically in patients with cancer [24]. Advantages of the Khorana scoring include its simplicity, and high negative predictive value, allowing physicians to exclude low-risk patients from thromboprophylaxis and related bleeding risks [5]. Limitations of the Khorana scoring involve a low positive predictive value, a need for further risk stratification (since many patients are classified as an intermediate risk), and no consistent validity in single sites of cancers [5]. Other clinical prediction scores, including the Vienna score, and the Protecht score, which modify the original Khorana score, serve as useful clinical prediction instruments for VTE risk in patients with cancer (Figure 1) [6, 7].
It should be noted that some randomized controlled trials (RCTs) have assessed the use of low molecular weight heparin (LMWH) for primary thromboprophylaxis in patients with cancer. In particular, semuloparin, an ultra- LMWH, was studied for efficacy and safety of thromboprophylaxis in a large group of unselected patients with locally advanced or metastatic cancer, receiving CHT [25]. After a median follow-up period of 3.5 months, VTE occurred in 1.2% of patients in the semuloparin group and in 3.4% of participants in the placebo group, and clinically relevant bleeding occurred in 2.8% of patients receiving semuloparin versus 2.0% of those receiving placebo [25]. When patients with cancer, at high risk of VTE (Khorana score of three or greater) were selected, thromboprophylaxis with dalteparin (LMWH) significantly reduced the VTE incidence (12% in the LMWH group versus 21% in the observation group), but the LMWH use resulted in a seven-fold increase risk of bleeding [26]. Therefore, routine use of LMWH for thromboprophylaxis in cancer patients was not recommended for unselected patients, according to practice guidelines (except from patients with pancreatic cancer receiving CHT) [27].
A patient’s poor performance status (e.g., according to the Eastern Cooperative Oncology Group (ECOG) ≥2) has been linked to an augmented risk of hypercoagulability. About 30% of patients with malignancies (e.g., in advanced stage), who were admitted to palliative care services suffered from a femoral deep vein thrombosis (DVT) [28]. Furthermore, surgical interventions, often associated with insertion of central venous catheters or other foreign devices, and immobilization, have increased the risk of DVT or PE among patients with malignancies. In addition, elderly age, preexisting motor dysfunctions, diagnosis of brain tumor (e.g., high-grade glioma) or gastric cancer were related to higher risk of VTE or PE occurrence in pre- and post-operative period [29, 30].
|
Table 1. Prophylaxis and treatment of thromboembolism in patients with cancer - current ASCO recommendations [19] |
||||
|
Validated scoring system for cancer-related VTE |
VTE prophylaxis for in-Pts |
VTE prophylaxis for high-risk out-Pts |
Prevention of VTE recurrence |
Practical implications for clinicians |
|
The Khorana score Pt variables in the assessment of VTE risk · site of ca, · platelet count, · leukocyte count, · Hb level, · BMI The KS is useful in anticoagulation planning for prophylaxis & treatment of Pts with ca-related VTE KS ≥ 3 indicate > VTE risk Exemplary KS scores:
Site of ca platelet count ≥350,000/mm3 – 1 leukocyte count >11,000/mm3 – 1 Hb <10 g/dL – 1 BMI ≥35kg/m2 – 1
|
Pts with active ca hospitalized for acute illness need thromboprophylaxis (except active bleeding or other contraindications) |
Before starting systemic CHT, thromboprophylaxis should be considered: · DOACs (apixaban, rivaroxaban) · or LMWH if there is no risk of · bleeding · pharmacologic interactions Thromboprophylaxis is more important for Pts receiving specific treatments for some ca, e.g., multiple myeloma treated with · thalidomide- or lenalidomide-based regimen, · dexamethasone, · or both Such Pts should receive ASA or LMWH, if they are at low risk for VTE; LMWH should be given for those who are at > VTE risk All Pts with ca undergoing major surgery should receive pharmacologic thromboprophylaxis with UFH or LMWH (unless there are bleeding contraindications) The prophylactic regimen should be initiated pre-op. and continued for at least 7-10 days post-op. In Pts at > VTE risk (e.g., with restricted mobility or obesity) LMWH should be extended for up to 4 weeks after major open or laparoscopic abdominal or pelvic surgery Mechanical methods against VTE can be used in addition to the pharmacologic agents |
For Pts with established VTE, · LMWH, · UFH, · fondaparinux, · or rivaroxaban should be used For long-term anticoagulation, · LMWH, · edoxaban, · or rivaroxaban for at least 6 ms are preferred due to improved efficacy over VKAs Beyond 6 ms, anticoagulation with LMWH, DOACs, or VKAs should be offered only to select Pts (e.g., with metastatic ca, or ongoing CHT) A vena cava filter should not be used in Pts with · chronic VTE · temporary contraindications to anticoagulantion (e.g., Pts requiring surgery) A vena cava filter can be an adjunct to anticoagulation for Pts with recurrent VTE Anticoagulation should be considered for Pts with primary or metastatic CNS ca & established VTE Incidental PE & DVT should be treated in the same way as symptomatic VTE In the absence of VTE, anticoagulants are not recommended to improve survival in Pts with ca Pts with ca should be evaluated for VTE risk initially (e.g., when starting systemic CHT), and then monitored periodically |
The routine use of pharmacologic thromboprophylaxis in all Pts with ca is not recommend Pts admitted for routine CHT or minor procedures (e.g., stem cell or bone marrow transplantation) do not require routine pharmacologic thromboprophylaxis Hospitalized Pts with active ca should be offered pharmacologic thromboprophylaxis Healthcare teams need to monitor possible AEs Pts need to be educated on · the risks for VTE · thromboprophy-laxis options e.g., when scheduled for major surgery, during hospitalization, and systemic CHT Physicians need to keep abreast with the ASCO guidelines & consider them in the evaluation of individual Pt’s VTE risk
|
|
Abbreviations: AEs, adverse effects; ASA, aspirin; ASCO, American Society of Clinical Oncology; ca, cancer; BMI, body mass index; CHT, chemotherapy; CNS, central nervous system; DOACs, direct oral anticoagulants; DVT, deep vein thrombosis; in-Pts, hospitalized patients; Hb, hemoglobin; KS, Khorana score; LMWH, low-molecular-weight heparin; ms, months; out-Pts, ambulatory patients; PE, pulmonary embolism; pre-op, preoperatively; post-op, postoperatively, Pts, patients; UFH, unfractionated heparin; VKAs, vitamin K antagonists; VTE, venous thromboembolism; |
||||
Similarly to many cytotoxic CHT agents, some targeted therapies reveal their pro-thrombotic properties in the oncology population. For instance, osimertinib (an epidermal growth factor receptor (EGFR) inhibitor, and lenvatinib (a tyrosine kinase receptor inhibitor (TKI), such as vascular endothelial growth factor (VEGF), fibroblast growth factor receptor, and platelet-derived growth factor (PDGF) receptor alpha) can contribute to an increased risk of VTE and pulmonary embolism (PE) [37, 38]. Lenvatinib has been investigated (in combination with carboplatin and paclitaxel) in patients with non-small-cell lung cancer, and has shown thrombotic adverse effects [38]. Likewise, in a study exploring treatment of patients with advanced cancer of the thyroid gland, lenvatinib has been noted to cause complications, such as PE or DVT in 3% of the trial participants [39].
Bevacizumab is an anti-angiogenesis monoclonal antibody, which targets vascular endothelial growth factor (VEGF) in the circulating blood. The combination of bevacizumab with irinotecan, fluorouracil, and leucovorin has improved outcomes in patients with colorectal cancer, but VTE episodes were higher among patients treated with bevacizumab compared to those receiving CHT alone (19.4% vs. 16.2%, respectively) [40]. A meta-analysis of twenty RCTs has revealed that the incidence of arterial thrombosis in patients using bevacizumab was 3.3%, and this risk was variable with different cancers (e.g., the highest relative risk of 3.72 was in patients with renal cell cancer, and the lowest of 1.89, was in patients with colorectal cancer) [ 41].
In the AVERT trial, patients with an active malignancy receiving CHT (with a Khorana score of 2 or above) were randomized to apixaban (2.5 mg twice daily) or placebo for six months [42]. In the intention-to-treat (ITT) analysis, the apixaban group had a decreased incidence of VTE compared to the placebo group (4.2% vs. 10.2%, respectively). [42]. However, the apixaban group had an increased incidence of major bleeding (3.5% vs. 1.8%) and clinically relevant non-major bleeding (7.3% vs. 5.5%) compared to the placebo group [42]. Otherwise, there was no difference in overall survival (OS) between these groups [42].
The CASSINI trial has examined the safety and efficacy of rivaroxaban (10 mg daily) in the prevention of cancer-related VTE [43].
Contrary to the AVERT (in which patients were not screened for VTE at the study screening period), participants in the CASSINI underwent venous duplex ultrasound screening for VTE in both legs, prior to entering the trial, and then, every two months, during the entire trial. Patients in whom an occult VTE was diagnosed were excluded from the study [43].
Moreover, CASSINI had a greater proportion of pancreatic cancer participants than AVERT (32% vs. 13%, respectively) and AVERT had slightly more patients with Khorana scores of 4 or greater than CASSINI (8.9% vs. 6.6%) [43].
In CASSINI, the ITT analysis found no significant reduction in VTE events in the rivaroxaban arm compared to placebo after 180 days and no increased risk of major bleeding [43]. However, in the on-treatment analysis, rivaroxaban significantly reduced VTE compared to placebo (2.6% vs. 6.4%). These findings suggest that in AVERT and CASSINI studies, the application of the Khorana risk score (e.g., of 2 or above), resulted in a more precise evaluation of low-dose DOAC vs. LMWH therapy, in comparison to the unselected population, assessed in the prior LMWH studies [44].
Furthermore, there is a scarcity of research exploring the efficacy and safety of extending anticoagulation for cancer-associated VTE beyond the first six months. Current guidelines usually recommend continuing therapy, if the malignancy is still present, or if the patient is still receiving anticancer treatment [19, 46]. The strongest evidence for treatment of cancer-related VTE with DOACs, beyond the initial six-months, has been derived from the Hokusai VTE Cancer trial, in which the patients were followed for up to one year [47]. The results of this trial support extended anticoagulation beyond the initial six-month treatment period [47]. In addition, a recent cohort study has also reported a reduced risk of VTE recurrence rate at one year, for patients treated with rivaroxaban compared to LMWH and VKA (warfarin) [48].
Thrombosis is a negative prognostic factor in many patients with cancer, who usually have a greater risk of VTE and bleeding. Early-onset of VTE, especially at the beginning of CHT is a poor prognostic factor for patients with metastatic cancer of the pancreas. Notably, among patients with cancer and acute VTE or PE, LMWH is more effective than an oral VKA for reducing the risk of recurrent VTE, without augmenting the risk of bleeding.
According to the updated ASCO clinical guidelines for the management of VTE in patients with cancer, the direct oral anticoagulants (DOACs), such as apixaban, edoxaban, and rivaroxaban represent a new opportunity for both the prevention and treatment of VTE in this population.
It should be underscored that each time, the application of thromboprophylaxis in a clinical setting should be done according to the dynamic proportions between the benefit of VTE reduction and the risk of bleeding. Further studies are necessary to elucidate the role of tumor genetic abnormalities in the VTE risk, across a wide spectrum of cancers. Hopefully, this may help refine the future risk stratification tools, which (in addition to the patient clinical context and preferences) should enable a more personalized selection of oncology patients for optimal VTE prevention and therapy.
Not applicable.
Funding
Not applicable.
Author contributions
KR contributes to the all of this paper.
Competing interests
All authors disclose no competing interests.
- Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC: Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122(10): 1712-1723.
- Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, Gates LE, et al: Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013, 31(17): 2189-2204.
- Nasser NJ, Fox J, Agbarya A: Potential Mechanisms of Cancer-Related Hypercoagulability. Cancers (Basel) 2020, 12(3): 566.
- Angelini DE, Radivoyevitch T, McCrae KR, Khorana AA: Bleeding incidence and risk factors among cancer patients treated with anticoagulation. Am J Hematol 2019, 94(7): 780-785.
- Khorana AA, Francis CW: Risk prediction of cancer-associated thrombosis: Appraising the first decade and developing the future. Thromb Res 2018, 164 Suppl 1: S70-S76.
- Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, Quehenberger P, Zielinski C, Pabinger I: Prediction of venous thromboembolism in cancer patients. Blood 2010, 116(24): 5377-5382.
- Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R: A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med 2012, 7(3): 291-292.
- Dickson BC: Venous thrombosis: on the history of Virchow’s triad. Univ Toronto Med J 2004, 81(3): 166-171.
- Nasser NJ, Na'amad M, Weinberg I, Gabizon AA: Pharmacokinetics of low molecular weight heparin in patients with malignant tumors. Anticancer Drugs 2015, 26(1): 106-111.
- Vlodavsky I, Gross-Cohen M, Weissmann M, Ilan N, Sanderson RD: Opposing Functions of Heparanase-1 and Heparanase-2 in Cancer Progression. Trends Biochem Sci 2018, 43(1): 18-31.
- Koliopanos A, Friess H, Kleeff J, Shi X, Liao Q, Pecker I, Vlodavsky I, Zimmermann A, Büchler MW: Heparanase expression in primary and metastatic pancreatic cancer. Cancer Res 2001, 61(12): 4655-4659.
- Cohen E, Doweck I, Naroditsky I, Ben-Izhak O, Kremer R, Best LA, Vlodavsky I, Ilan N: Heparanase is overexpressed in lung cancer and correlates inversely with patient survival. Cancer 2008, 113(5): 1004-1011.
- Li HL, Gu J, Wu JJ, Ma CL, Yang YL, Wang HP, Wang J, Wang Y, Chen C, Wu HY: Heparanase mRNA and Protein Expression Correlates with Clinicopathologic Features of Gastric Cancer Patients: a Meta- analysis. Asian Pac J Cancer Prev 2015, 16(18): 8653-8658.
- Chiu JJ, Chien S: Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 2011, 91(1): 327-387.
- Rak J, Milsom C, Yu J: Tissue factor in cancer. Curr Opin Hematol 2008, 15(5): 522-528.
- Abbonante V, Chitalia V, Rosti V, Leiva O, Matsuura S, Balduini A, Ravid K: Upregulation of lysyl oxidase and adhesion to collagen of human megakaryocytes and platelets in primary myelofibrosis. Blood 2017, 130(6): 829-831.
- Nadir Y, Brenner B, Zetser A, Ilan N, Shafat I, Zcharia E, Goldshmidt O, Vlodavsky I: Heparanase induces tissue factor expression in vascular endothelial and cancer cells. J Thromb Haemost 2006, 4(11): 2443-2451.
- Ay C, Pabinger I, Cohen AT: Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost 2017, 117(2): 219-230.
- Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW, et al: Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2020, 38(5): 496-520.
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH: Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007, 110(10): 2339-2346.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR: Malignancies, prothrombotic mutations, and the risk of venous thrombosis. Jama 2005, 293(6): 715-722.
- Khorana AA, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, Lee AY: Tissue Factor As a Predictor of Recurrent Venous Thromboembolism in Malignancy: Biomarker Analyses of the CATCH Trial. J Clin Oncol 2017, 35(10): 1078-1085.
- Ay C, Simanek R, Vormittag R, Dunkler D, Alguel G, Koder S, Kornek G, Marosi C, Wagner O, Zielinski C, et al: High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood 2008, 112(7): 2703-2708.
- Khorana AA, Rao MV: Approaches to risk-stratifying cancer patients for venous thromboembolism. Thromb Res 2007, 120 Suppl 2: S41-50.
- Agnelli G, George DJ, Kakkar AK, Fisher W, Lassen MR, Mismetti P, Mouret P, Chaudhari U, Lawson F, Turpie AG: Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 2012, 366(7): 601-609.
- Khorana AA, Francis CW, Kuderer NM, Carrier M, Ortel TL, Wun T, Rubens D, Hobbs S, Iyer R, Peterson D, et al: Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: A randomized trial. Thromb Res 2017, 151: 89-95.
- Farge D, Bournet B, Conroy T, Vicaut E, Rak J, Zogoulous G, Barkun J, Ouaissi M, Buscail L, Frere C: Primary Thromboprophylaxis in Pancreatic Cancer Patients: Why Clinical Practice Guidelines Should Be Implemented. Cancers (Basel) 2020, 12(3): 618.
- White C, Noble SIR, Watson M, Swan F, Allgar VL, Napier E, Nelson A, McAuley J, Doherty J, Lee B, et al: Prevalence, symptom burden, and natural history of deep vein thrombosis in people with advanced cancer in specialist palliative care units (HIDDen): a prospective longitudinal observational study. Lancet Haematol 2019, 6(2): e79-e88.
- Chaichana KL, Pendleton C, Jackson C, Martinez-Gutierrez JC, Diaz-Stransky A, Aguayo J, Olivi A, Weingart J, Gallia G, Lim M, et al: Deep venous thrombosis and pulmonary embolisms in adult patients undergoing craniotomy for brain tumors. Neurol Res 2013, 35(2): 206-211.
- Osaki T, Saito H, Fukumoto Y, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, Sato K, Hirooka Y, et al: Risk and incidence of perioperative deep vein thrombosis in patients undergoing gastric cancer surgery. Surg Today 2018, 48(5): 525-533.
- Khorana AA, Dalal M, Lin J, Connolly GC: Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013, 119(3): 648-655.
- Seng S, Liu Z, Chiu SK, Proverbs-Singh T, Sonpavde G, Choueiri TK, Tsao CK, Yu M, Hahn NM, Oh WK, et al: Risk of venous thromboembolism in patients with cancer treated with Cisplatin: a systematic review and meta-analysis. J Clin Oncol 2012, 30(35): 4416-4426.
- Starling N, Rao S, Cunningham D, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR: Thromboembolism in patients with advanced gastroesophageal cancer treated with anthracycline, platinum, and fluoropyrimidine combination chemotherapy: a report from the UK National Cancer Research Institute Upper Gastrointestinal Clinical Studies Group. J Clin Oncol 2009, 27(23): 3786-3793.
- Moore RA, Adel N, Riedel E, Bhutani M, Feldman DR, Tabbara NE, Soff G, Parameswaran R, Hassoun H: High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol 2011, 29(25): 3466-3473.
- Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, Dimitrov NV, Wolmark N, Wickerham DL, Fisher ER, et al: A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med 1989, 320(8): 479-484.
- Fisher B, Dignam J, Wolmark N, DeCillis A, Emir B, Wickerham DL, Bryant J, Dimitrov NV, Abramson N, Atkins JN, et al: Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst 1997, 89(22): 1673-1682.
- Shiroyama T, Hayama M, Satoh S, Nasu S, Tanaka A, Morita S, Morishita N, Suzuki H, Okamoto N, Hirashima T: Successful retreatment with osimertinib after osimertinib-induced acute pulmonary embolism in a patient with lung adenocarcinoma: A case report. Respir Med Case Rep 2017, 20: 25-27.
- Nishio M, Horai T, Horiike A, Nokihara H, Yamamoto N, Takahashi T, Murakami H, Yamamoto N, Koizumi F, Nishio K, et al: Phase 1 study of lenvatinib combined with carboplatin and paclitaxel in patients with non-small-cell lung cancer. Br J Cancer 2013, 109(3): 538-544.
- Cabanillas ME, Schlumberger M, Jarzab B, Martins RG, Pacini F, Robinson B, McCaffrey JC, Shah MH, Bodenner DL, Topliss D, et al: A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: A clinical outcomes and biomarker assessment. Cancer 2015, 121(16): 2749-2756.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004, 350(23): 2335-2342.
- Ranpura V, Hapani S, Chuang J, Wu S: Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol 2010, 49(3): 287-297.
- Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, Kuruvilla P, Hill D, Spadafora S, Marquis K, et al: Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N Engl J Med 2019, 380(8): 711-719.
- Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, Streiff MB, Garcia DA, Liebman HA, Belani CP, et al: Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med 2019, 380(8): 720-728.
- Agnelli G: Direct Oral Anticoagulants for Thromboprophylaxis in Ambulatory Patients with Cancer. N Engl J Med 2019, 380(8): 781-783.
- Al-Samkari H, Connors JM: Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy. Blood Adv 2019, 3(22): 3770-3779.
- Streiff MB, Holmstrom B, Angelini D, Ashrani A, Bockenstedt PL, Chesney C, Fanikos J, Fenninger RB, Fogerty AE, Gao S, et al: NCCN Guidelines Insights: Cancer-Associated Venous Thromboembolic Disease, Version 2.2018. J Natl Compr Canc Netw 2018, 16(11): 1289-1303.
- Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, et al: Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med 2018, 378(7): 615-624.
- Streiff MB, Milentijevic D, McCrae K, Yannicelli D, Fortier J, Nelson WW, Laliberté F, Crivera C, Lefebvre P, Schein J, et al: Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol 2018, 93(5): 664-671.
Asia-Pacific Journal of Oncology
print ISSN: 2708-7980, online ISSN: 2708-7999
 Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript