Review | Open Access
Review of immunotherapy in non-small cell lung cancer: mechanisms, clinical applications, and future prospects
Palwasha Habib1
1Department of Medicine, Mohtarma Benazir Bhutto Shaheed Medical College, Mirpur, Azad Jammu and Kashmir (AJK).
Correspondence: Palwasha Habib (Department of Medicine, Mohtarma Benazir Bhutto Shaheed Medical College, Mirpur, AJK; Email: drpalwashahabib786@gmail.com).
Asia-Pacific Journal of Oncology 2024, 5: 39-45. https://doi.org/10.32948/ajo.2024.09.08
Received: 15 Aug 2024 | Accepted: 08 Sep 2024 | Published online: 11 Sep 2024
Key words non-small cell lung cancer (NSCLC), PD-1/PD-L1 pathway, CTLA-4, oncolytic virus, pathway, immunotherapy
PD-1/PD-L1 pathway
The PD-1/PD-L1 pathway is one of the crucial immune regulation pathways. The two key proteins involved in this axis include the receptor-that is, programmed cell death protein 1 (PD-1) and its ligand, which is a protein known as Programmed death-ligand 1 (PD-L1). PD-1 is a receptor expressed on the surface of activated T cells, B cells, and some other immune cells [9]. The ligand PD-L1 is expressed by a wide array of cell types, including tumor cells and antigen-presenting cells such as dendritic cells and macrophages [7]. The pathway plays a critical role in balancing the immune response, preventing autoimmune responses. However, within the tumor microenvironment, this mechanism is often exploited for immune surveillance evasion [10]. In fact, the pathway of PD-1/PD-L1 plays an important role in immune escape mechanisms in NSCLC. The tumor cells can upregulate the expression of PD-L1 to interact with the PD-1 receptor on T cells, which inhibits the activity of T cells and thus allows tumor cells to escape from immune surveillance [11]. This interaction provides a new approach for the treatment of non-small cell lung cancer (NSCLC). PD-1/PD-L1 inhibitors restore T cell activity by blocking the interaction between PD-1 and PD-L1, helping the immune system recognize and destroy tumor cells. PD-1 inhibitors such as nivolumab and pembrolizumab block their interaction with PD-L1 by binding to PD-1 [12]; PD-L1 inhibitors such as atezolizumab bind to PD-L1, preventing its binding to PD-1 and relieving its inhibition on T cells [13].
CTLA-4 pathway
Another important immune checkpoint pathway is the CTLA-4 pathway, which modulates the amplitude of the immune activity and impairs autoimmune reactions. The pathway includes CTLA-4 and B7 molecules [14]. In NSCLC, the tumor cells can facilitate CTLA-4 expression or enhance the activity of the B7 molecule to restrain further T cell function, promoting immune escape for tumor cells. Engagement of the CTLA-4 pathway inhibits T cells from fully exerting their antitumor function-promoting tumor growth and spread [15]. CTLA-4 inhibitors block the interaction between CTLA-4 and B7 molecules, releasing the inhibition on T cells, thereby restoring and enhancing their antitumor activity. Specifically, CTLA-4 inhibitors like Ipilimumab bind to CTLA-4, preventing its interaction with B7 molecules, and enhancing T cell activation and proliferation [16]. CTLA-4 inhibitors can also increase the activity of antigen-presenting cells, leading to the presentation of more tumor antigens to T cells and enhancing the immune response.
Oncolytic viruses
Oncolytic viruses, as a novel immunotherapy mechanism, use naturally occurring or genetically engineered viruses to selectively infect and kill tumor cells [17]. These viruses not only directly destroy tumor cells but also activate the host immune system to combat the tumor [18]. NSCLC cells express specific receptors that can be recognized and bound by oncolytic viruses. For example, some adenoviruses can selectively infect NSCLC cells by recognizing the Coxsackievirus and Adenovirus Receptor (CAR) on their surface [19]. Oncolytic viruses have the advantage of replicating and spreading more easily in NSCLC cells, whose cells typically have defective antiviral defences. Oncolytic viruses once inside NSCLC cells replicate, release new virus particles, break the cell membrane, and lyses and kill the cell. Oncolytic viruses exert their direct oncolytic effect, but can also stimulate the host immune response against NSCLC cells [20].
Chimeric antigen receptor T-cells (CAR-T cells)
Immunotherapy in the form of Chimeric Antigen Receptor T-cell Therapy is a very sophisticated form in which the patient’s T cells are genetically altered to express chimeric antigen receptors (CARs) to recognize and kill tumour cells. To date, this therapeutic modality has been quite successful in haematological malignancies and is being studied for its applicability in solid tumours, including NSCLC [21], [22]. The major process is peripheral blood collection and sorting of T cells; the addition of a gene encoding for a single chain antibody (scFv) that recognizes tumour-specific antigens, including a chimeric antigen receptor (CAR)-a chain that activates the T cell signalling domain. Viral vectors (mostly lentiviruses or retroviruses) are used to deliver this gene to the T cells [23]. The modified CAR-T cells are expanded in vitro and then intravenously injected back into the body. In vivo, through the specificity of the CAR structure, the modified CAR-T cells bind to antigens on the surface of tumor cells. After binding to the tumor cells, the CAR-T cells are activated to release cytotoxic particles (such as perforin and granzyme), which kill tumor cells [24].
Immunotherapy for non-small cell lung cancer (NSCLC) mainly involves several mechanisms: PD-1/PD-L1 pathway inhibitors restore T cell immune function by blocking the interaction between tumor cells and T cells; CTLA-4 inhibitors enhance immune responses by relieving inhibition of T cells; Oncolytic viruses selectively infect and kill tumor cells while activating the immune system; CAR-T cell therapy involves genetically modifying T cells to recognize and kill tumor cells. These methods collectively enhance the immune system's attack on tumors, providing new directions for NSCLC treatment.
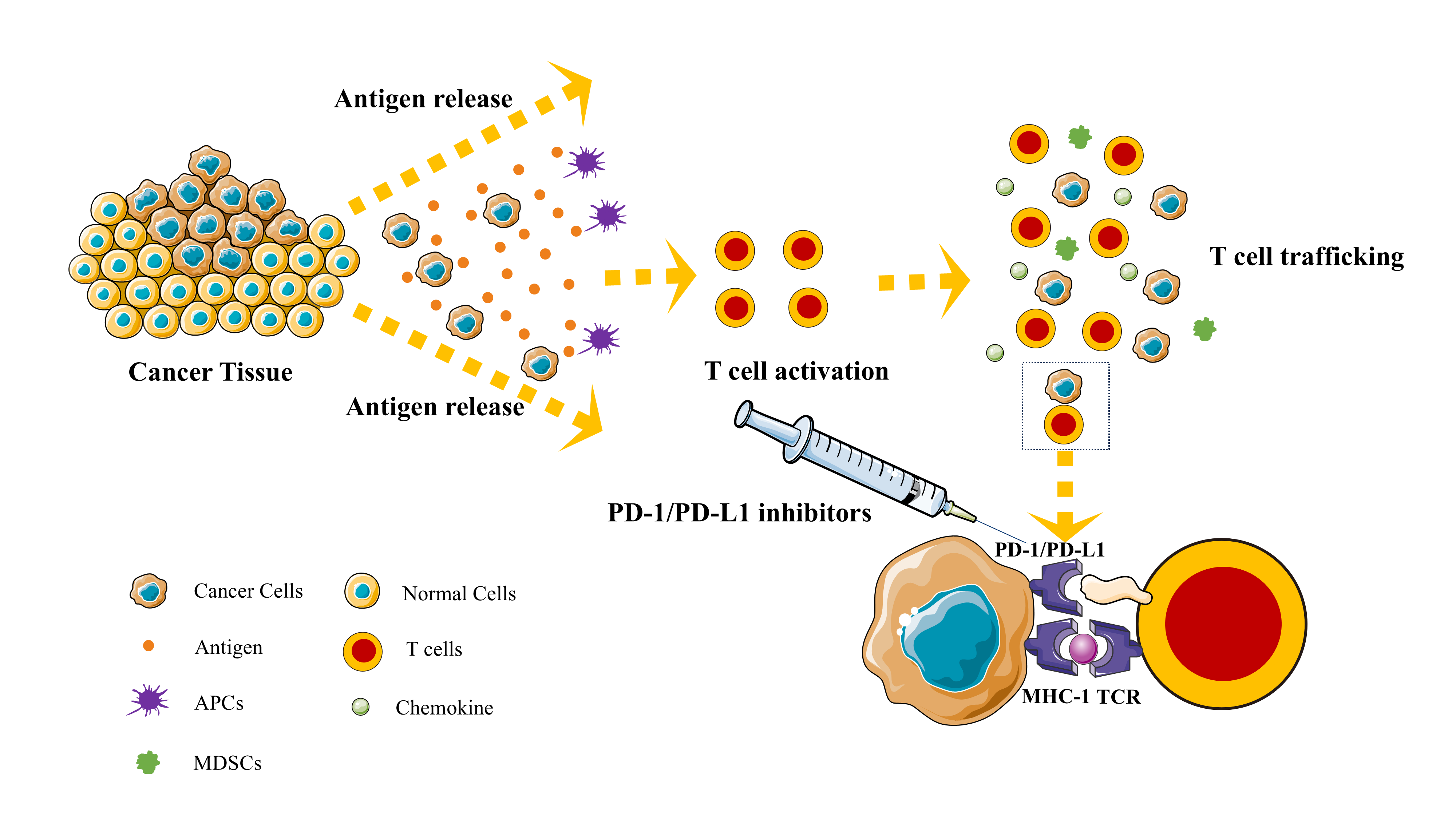 Figure 1. Plot of the mechanism of immunotherapy for non-small cell lung cancer (NSCLC).
Figure 1. Plot of the mechanism of immunotherapy for non-small cell lung cancer (NSCLC).
Nivolumab (Opdivo)
Nivolumab (Opdivo) is the first PD-1 inhibitor approved for the treatment of NSCLC. It is under investigation in two pivotal clinical trials; CheckMate-017 and CheckMate-057 were designed to test Nivolumab against docetaxel in patients with advanced squamous NSCLC [27], [28]. Both studies demonstrated that the median overall survival(OS) in the Nivolumab group was 9.2 months, while in the docetaxel group, it was 6.0 months. Whereas the overall survival rate at 1 year was 42% in the Nivolumab group, it was 24% in the docetaxel group. These clinical trials have underlined the great step forward that Nivolumab has achieved in treating NSCLC by highly increasing both overall survival (OS) and progression-free survival (PFS) of patients with advanced NSCLC.
New immune checkpoints
The enormous success of therapies with PD-1/PD-L1 and CTLA-4 inhibitors in cancer treatment has shifted the investigations to other novel immune checkpoints to overcome the inefficacy of the existing therapies. LAG-3 (lymphocyte activation gene 3) is a receptor expressed on activated T cells, natural killer (NK) cells, and some B cells, typically inhibiting T cell function by binding to MHC class II molecules. Preliminary studies have shown that the combination of LAG-3 inhibitors and PD-1 inhibitors can overcome resistance to PD-1 monotherapy and improve efficacy [29]. TIM-3 (T-cell immunoglobulin and mucin 3) is expressed in T cells, NK cells, and monocytes as a negative regulatory molecule that inhibits T cell function. When TIM-3 binds to its ligands (such as Galectin-9), it can induce immune suppression signals [30]. TIM-3 inhibitors may release T cell inhibition through this mechanism and enhance the immune system's anti-tumor ability, especially in PD-1-resistant patients [31]. TIGIT (T-cell immunoglobulin and ITIM domain) is expressed on T cells and NK cells, binds to CD155, transmits inhibitory signals, reduces the activity of T cells and NK cells, and can also compete with CD226 to bind to CD155, thereby inhibiting immune activation [32]. Preliminary studies have shown that in some patients, the combination of TIGIT inhibitors and PD-L1 inhibitors (such as Atezolizumab) exhibits enhanced anti-tumor activity. B7-H3 (CD276) is a molecule expressed on multiple cell types, including tumor cells, that participates in immune suppression and plays a role in tumor immune escape [33]. VISTA (V-domain Ig suppressor of T cell activation) is a negative immune regulatory molecule expressed on myeloid cells and some T cells [34]. It suppresses T cell activation and proliferation by binding to its ligands. As a new immune checkpoint, VISTA inhibitors are undergoing early clinical trials to explore their potential in cancer treatment [35], [36]. Research into new immune checkpoints is rapidly in development with a view to overcoming the limitations of current immunotherapies and offering patients more treatment options [37]. Further investigation of these novel immune checkpoints and their roles within the tumor microenvironment will continue to lead to the development of new inhibitors and combination therapies aimed at improving therapeutic efficacy in NSCLC and other cancers.
Oncolytic viruses
Four strains of oncolytic viruses have been primarily developed: Oncorine (H101) [38], Talimogene laherparepvec (T-VEC) [39], Reovirus [40], and measles virus [41]. H101 is a genetically modified adenovirus and has become the first approved in China for the treatment of head and neck squamous cell carcinoma. Its research in NSCLC has been promising, especially when combined with chemotherapy, enhancing the efficacy of the treatment. T-VEC is a genetically modified herpes simplex virus (HSV) used for the treatment of unresectable melanoma; its possible use in NSCLC is being explored [42]. Early-phase clinical trials showed that T-VEC induces an immune response in NSCLC patients; when combined with PD-1 inhibitors, it has synergistic effects. Reovirus is a naturally occurring virus that selectively infects and kills tumor cells. Clinical trials in NSCLC have demonstrated good safety of Reovirus with potential efficacy, especially in combination with immune checkpoint inhibitors. Genetic modification of the measles virus is used for the treatment of a variety of cancers including NSCLC. Preclinical studies using the measles virus demonstrate that it is selectively capable of infecting and destroying NSCLC cells, inducing a potent anti-tumor immune response.
Biomarkers
Identifying and applying biomarkers are important in identifying and applying the prediction of treatment response and optimization of treatment plans for improving efficacy in immunotherapies for non-small cell lung cancer (NSCLC). Tumor mutation burden (TMB) is defined as the total number of mutations present in the tumor genome per million base pairs. High TMB usually correlates with the generation of neo-antigens, which in general would more potently induce an immune response. Therefore, TMB is also considered an important biomarker for the prediction of responses to immune therapy [43]. CheckMate-227 was a clinical trial for testing the efficiency of Nivolumab (PD-1 inhibitor) in combination with Ipilimumab (CTLA-4 inhibitor) in high TMB NSCLC. Results of the study proved that in the case of high TMB (≥10 mutations/Mb) progression-free survival (PFS) and overall survival (OS) rates were significantly longer following combination treatment, demonstrating that high TMB is associated with better outcomes [44]. Thus, testing of TMB could be one of the important criteria for the selection of appropriate candidates for immunotherapy.
Circulating tumor DNA (ctDNA) is a tumor-derived free DNA fragment released into the bloodstream and has become an emerging biomarker in NSCLC immunotherapy. ctDNA has demonstrated significant potential in cancer detection and monitoring, particularly in NSCLC immunotherapy, where it offers dynamic, non-invasive insights into tumor characteristics and treatment response [45]. Currently, the primary methods for detecting ctDNA include digital PCR (dPCR), next-generation sequencing (NGS), and BEAMing technology. Research has shown that patients with high levels of ctDNA before immunotherapy experience a significant decrease in ctDNA levels after treatment, indicating a more favorable treatment response and serving as a predictor of treatment outcomes. Efficacy monitoring can also be conducted, and by regularly monitoring ctDNA levels, the effectiveness of immunotherapy can be evaluated in real-time. An increase in ctDNA levels implies that an increase in levels may signify the progress or recurrence of a disease, with early intervention resulting in better outcomes for the patients [46]. Analyzing ctDNA may also be used to monitor new drug-resistant mutations so that adjustments in treatment can be made. Compared with traditional methods of tissue biopsy, testing of ctDNA can be done through simple blood extraction and hence reduces the pain and risk for patients. It provides an indication of the heterogeneity of tumors throughout the body and offers more complete genomic information compared with single tissue biopsy [47]. While detection technologies for ctDNA continue to improve, increased sensitivity and specificity, especially with respect to the detection of low-frequency mutations, is still highly desirable. Detection processes and methods of analysis need to be standardized to ensure that the results from different laboratories are comparable. Large-scale clinical investigation of the effectiveness and reliability of the ctDNA prediction for responses to immune therapy and monitoring drug resistance will be further required in future studies.
Neoantigen refers to a kind of antigen that is produced by a special mutation of tumor cells and is not expressed in normal cells. These neoantigens can be recognized by the immune system, inducing a potent anti-tumor response. Neoantigen load means the amount of neoantigen produced in tumor cells, and the higher neoantigen load usually had better immune therapy response [48]. The main mechanism of action thereby involves neoantigen presentation to T cells through major histocompatibility complex (MHC) molecules, thus stimulating T cells to recognize and attack tumor cells. Neoantigen presentation increases the possibility of tumor recognition by the immune system and, as a result, enhances the immune surveillance function. The KEYNOTE-010 clinical trial evaluated the efficacy of Pembrolizumab (PD-1 inhibitor) in PD-L1 positive NSCLC patients, indicating a positive correlation between neoantigen burden and Pembrolizumab treatment response, with patients with high antigen burden showing better treatment outcomes [49]. This indicates that neoantigen burden can be used as a biomarker for selecting suitable immunotherapy patients, and patients with a high antigen burden are more likely to benefit from immune checkpoint inhibitor therapy. In the future, it can be combined with other biomarkers such as TMB, PD-L1 expression, and TILs to improve prediction accuracy.
|
Table 1. Characteristics of immune checkpoint inhibitors in the treatment of NSCLC. |
||||
|
Drug/Therapy |
Target |
Primary indication |
Combination therapy strategy |
Major efficacy |
|
Nivolumab (Opdivo) |
PD-1 inhibitor |
Advanced or metastatic NSCLC patients with progression after chemotherapy |
Combined with Ipilimumab |
ORR: 20%-30% |
|
Pembrolizumab (Keytruda) |
PD-1 inhibitor |
Advanced or metastatic NSCLC patients with high PD-L1 expression, as a first-line therapy |
Combined with chemotherapy |
ORR: 40%-50%( In patients with PD-L1 expression ≥50%) |
|
Atezolizumab (Tecentriq) |
PD-L1 inhibitor |
Advanced or metastatic NSCLC patients with progression after chemotherapy |
Combined with chemotherapy and bevacizumab |
ORR: 15%-20% |
|
Ipilimumab (Yervoy) |
CTLA-4 inhibitor |
Primarily for melanoma treatment, shows potential in NSCLC |
Combined with Nivolumab |
ORR: 15%-20% |
In summary, although immunotherapy has achieved significant efficacy in the treatment of non-small cell lung cancer (NSCLC), its side effects cannot be ignored, mainly including rash, itching, pneumonia, thyroid dysfunction, which may reduce the quality of life of patients. Early detection, timely intervention, and individualized management plans are crucial to reduce the occurrence of these side effects. These measures can effectively reduce side effects and ensure that patients receive maximum efficacy and benefits from immunotherapy.
No applicable.
Availability of data and materials
Data and materials are available on request from the authors.
Ethical policy
Not applicable.
Author contributions
PH conceptualized, designed, conducted research, wrote the draft and approve the final manuscript.
Competing interests
The authors have no conflicts of interest regarding the publication of this paper.
Funding
None.
- Jonna S, Subramaniam DS: Molecular diagnostics and targeted therapies in non-small cell lung cancer (NSCLC): an update. Discov Med 2019, 27(148): 167-170.
- Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK: Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014, 14(8): 535-546.
- Passaro A, Mok TSK, Attili I, Wu YL, Tsuboi M, de Marinis F, Peters S: Adjuvant Treatments for Surgically Resected Non-Small Cell Lung Cancer Harboring EGFR Mutations: A Review. JAMA Oncol 2023, 9(8): 1124-1131.
- Rossi A, Di Maio M: Platinum-based chemotherapy in advanced non-small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther 2016, 16(6): 653-660.
- Yu Y, Zeng D, Ou Q, Liu S, Li A, Chen Y, Lin D, Gao Q, Zhou H, Liao W et al: Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis. JAMA Netw Open 2019, 2(7): e196879.
- Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, Mukherjee A, Paul MK: Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer 2023, 22(1): 40.
- Han Y, Liu D, Li L: PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 2020, 10(3): 727-742.
- Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK: PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol 2017, 8: 561.
- Ai L, Xu A, Xu J: Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv Exp Med Biol 2020, 1248: 33-59.
- Salmaninejad A, Valilou SF, Shabgah AG, Aslani S, Alimardani M, Pasdar A, Sahebkar A: PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J Cell Physiol 2019, 234(10): 16824-16837.
- Xia L, Liu Y, Wang Y: PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions. Oncologist 2019, 24(Suppl 1): S31-s41.
- Sundar R, Cho BC, Brahmer JR, Soo RA: Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol 2015, 7(2): 85-96.
- Ryu R, Ward KE: Atezolizumab for the First-Line Treatment of Non-small Cell Lung Cancer (NSCLC): Current Status and Future Prospects. Front Oncol 2018, 8: 277.
- Rowshanravan B, Halliday N, Sansom DM: CTLA-4: a moving target in immunotherapy. Blood 2018, 131(1): 58-67.
- Hosseini A, Gharibi T, Marofi F, Babaloo Z, Baradaran B: CTLA-4: From mechanism to autoimmune therapy. Int Immunopharmacol 2020, 80: 106221.
- Naimi A, Mohammed RN, Raji A, Chupradit S, Yumashev AV, Suksatan W, Shalaby MN, Thangavelu L, Kamrava S, Shomali N et al: Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun Signal 2022, 20(1): 44.
- Mondal M, Guo J, He P, Zhou D: Recent advances of oncolytic virus in cancer therapy. Hum Vaccin Immunother 2020, 16(10): 2389-2402.
- Malhotra J, Kim ES: Oncolytic Viruses and Cancer Immunotherapy. Curr Oncol Rep 2023, 25(1): 19-28.
- Niemann J, Kühnel F: Oncolytic viruses: adenoviruses. Virus Genes 2017, 53(5): 700-706.
- Masemann D, Meissner R, Schied T, Lichty BD, Rapp UR, Wixler V, Ludwig S: Synergistic anti-tumor efficacy of oncolytic influenza viruses and B7-H3 immune- checkpoint inhibitors against IC-resistant lung cancers. Oncoimmunology 2021, 10(1): 1885778.
- Qu J, Mei Q, Chen L, Zhou J: Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives. Cancer Immunol Immunother 2021, 70(3): 619-631.
- Kim YJ, Li W, Zhelev DV, Mellors JW, Dimitrov DS, Baek DS: Chimeric antigen receptor-T cells are effective against CEACAM5 expressing non-small cell lung cancer cells resistant to antibody-drug conjugates. Front Oncol 2023, 13: 1124039.
- Martinez M, Moon EK: CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front Immunol 2019, 10: 128.
- Kosti P, Maher J, Arnold JN: Perspectives on Chimeric Antigen Receptor T-Cell Immunotherapy for Solid Tumors. Front Immunol 2018, 9: 1104.
- Tang S, Qin C, Hu H, Liu T, He Y, Guo H, Yan H, Zhang J, Tang S, Zhou H: Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Progress, Challenges, and Prospects. Cells 2022, 11(3): 320.
- Wu Z, Man S, Sun R, Li Z, Wu Y, Zuo D: Recent advances and challenges of immune checkpoint inhibitors in immunotherapy of non-small cell lung cancer. Int Immunopharmacol 2020, 85: 106613.
- Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro Carpeno J, Pluzanski A, Arrieta O, Frontera OA, Chiari R et al: Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol 2021, 39(7): 723-733.
- Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L et al: Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017, 35(35): 3924-3933.
- Aggarwal V, Workman CJ, Vignali DAA: LAG-3 as the third checkpoint inhibitor. Nat Immunol 2023, 24(9): 1415-1422.
- Sauer N, Janicka N, Szlasa W, Skinderowicz B, Kołodzińska K, Dwernicka W, Oślizło M, Kulbacka J, Novickij V, Karłowicz-Bodalska K: TIM-3 as a promising target for cancer immunotherapy in a wide range of tumors. Cancer Immunol Immunother 2023, 72(11): 3405-3425.
- Gomes de Morais AL, Cerdá S, de Miguel M: New Checkpoint Inhibitors on the Road: Targeting TIM-3 in Solid Tumors. Curr Oncol Rep 2022, 24(5): 651-658.
- Harjunpää H, Guillerey C: TIGIT as an emerging immune checkpoint. Clin Exp Immunol 2020, 200(2): 108-119.
- Zhou WT, Jin WL: B7-H3/CD276: An Emerging Cancer Immunotherapy. Front Immunol 2021, 12: 701006.
- Yuan L, Tatineni J, Mahoney KM, Freeman GJ: VISTA: A Mediator of Quiescence and a Promising Target in Cancer Immunotherapy. Trends Immunol 2021, 42(3): 209-227.
- Wu C, Cao X, Zhang X: VISTA inhibitors in cancer immunotherapy: a short perspective on recent progresses. RSC Med Chem 2021, 12(10): 1672-1679.
- Shekari N, Shanehbandi D, Kazemi T, Zarredar H, Baradaran B, Jalali SA: VISTA and its ligands: the next generation of promising therapeutic targets in immunotherapy. Cancer Cell Int 2023, 23(1): 265.
- Kong X: Discovery of New Immune Checkpoints: Family Grows Up. Adv Exp Med Biol 2020, 1248: 61-82.
- Liang M: Oncorine, the World First Oncolytic Virus Medicine and its Update in China. Curr Cancer Drug Targets 2018, 18(2): 171-176.
- Salloum A, Koblinski J, Bazzi N, Zeitouni NC: Talimogene Laherparepvec in Non-Melanoma Cancers. J Clin Aesthet Dermatol 2021, 14(11): 18-25.
- Kumar V, Giacomantonio MA, Gujar S: Role of Myeloid Cells in Oncolytic Reovirus-Based Cancer Therapy. Viruses 2021, 13(4): 654.
- Blechacz B, Russell SJ: Measles virus as an oncolytic vector platform. Curr Gene Ther 2008, 8(3): 162-175.
- Shalhout SZ, Miller DM, Emerick KS, Kaufman HL: Therapy with oncolytic viruses: progress and challenges. Nat Rev Clin Oncol 2023, 20(3): 160-177.
- Jardim DL, Goodman A, de Melo Gagliato D, Kurzrock R: The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell 2021, 39(2): 154-173.
- Paz-Ares LG, Ramalingam SS, Ciuleanu TE, Lee JS, Urban L, Caro RB, Park K, Sakai H, Ohe Y, Nishio M et al: First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial. J Thorac Oncol 2022, 17(2): 289-308.
- Pessoa LS, Heringer M, Ferrer VP: ctDNA as a cancer biomarker: A broad overview. Crit Rev Oncol Hematol 2020, 155: 103109.
- Stadler JC, Belloum Y, Deitert B, Sementsov M, Heidrich I, Gebhardt C, Keller L, Pantel K: Current and Future Clinical Applications of ctDNA in Immuno-Oncology. Cancer Res 2022, 82(3): 349-358.
- Snyder A, Morrissey MP, Hellmann MD: Use of Circulating Tumor DNA for Cancer Immunotherapy. Clin Cancer Res 2019, 25(23): 6909-6915.
- Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong F, Guo C, Wu X, Li Y, Li X et al: Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer 2019, 18(1): 128.
- Herbst RS, Garon EB, Kim DW, Cho BC, Gervais R, Perez-Gracia JL, Han JY, Majem M, Forster MD, Monnet I et al: Five Year Survival Update From KEYNOTE-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J Thorac Oncol 2021, 16(10): 1718-1732.
- Tan S, Li D, Zhu X: Cancer immunotherapy: Pros, cons and beyond. Biomed Pharmacother 2020, 124: 109821.
- Ke W, Zhang L, Dai Y: The role of IL-6 in immunotherapy of non-small cell lung cancer (NSCLC) with immune-related adverse events (irAEs). Thorac Cancer 2020, 11(4): 835-839.
- Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF, Long NM et al: Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol Res 2018, 6(9): 1093-1099.
- Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, Rutkowski P, Gogas HJ, Lao CD, De Menezes JJ et al: Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med 2022, 386(1): 24-34.
- Cheng W, Kang K, Zhao A, Wu Y: Dual blockade immunotherapy targeting PD-1/PD-L1 and CTLA-4 in lung cancer. J Hematol Oncol 2024, 17(1): 54.
- Dao J, Conway PJ, Subramani B, Meyyappan D, Russell S, Mahadevan D: Using cfDNA and ctDNA as Oncologic Markers: A Path to Clinical Validation. Int J Mol Sci 2023, 24(17): 13219.
- Mamdani H, Matosevic S, Khalid AB, Durm G, Jalal SI: Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front Immunol 2022, 13: 823618.
Asia-Pacific Journal of Oncology
print ISSN: 2708-7980, online ISSN: 2708-7999
 Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript