Review | Open Access
Current approaches to the management of neuroendocrine breast carcinoma (NEBC): a review
Mohsen S. Ali1
1Department of Internal Medicine, Faculty of Medicine, Thamar University, Dhamar, Yemen.
Correspondence: Mohsen S. Ali (Department of Internal Medicine, Faculty of Medicine, Thamar University, Dhamar, Yemen; Email: Moh_aljumaini@hotmail.com).
Asia-Pacific Journal of Oncology 2024, 5: 85-94. https://doi.org/10.32948/ajo.2024.11.25
Received: 10 Oct 2024 | Accepted: 19 Nov 2024 | Published online: 27 Nov 2024
Key words neuroendocrine breast cancer, breast carcinoma, neuroendocrine tumor, small-cell breast cancer, breast
Neuroendocrine neoplasms (NEN) possess characteristics that differentiate them from other solid tumors [4]. Distributed throughout the body, neuroendocrine cells possess dual characteristics: a morphology akin to nerve cells and biological functions resembling those of endocrine cells [5]. NEBC, alternatively termed breast carcinoma with neuroendocrine differentiation, is a heterogeneous group of relatively sporadic tumors that accounts for 2–5% of all invasive breast cancers [6]. The variation in reported statistics may be related to the absence of standardized histopathological and immunohistochemical diagnostic standards, as neuroendocrine biomarkers are rarely used in the diagnosis of breast tumor [7]. It is a rare and poorly differentiated form of breast cancer that is often underestimated [8, 9]. However, the prognostic significance of these markers remains unclear. Due to its low prevalence, there is also a lack of information regarding the most effective treatment of NEBC. This review highlights the existing knowledge regarding the histopathology and immunohistochemical characteristics, as well as the treatment and prognosis of NEBC.
Breast neuroendocrine tumors (NETs) were recognized as a separate breast component by the WHO Classification (3rd Edition) until 2003, when NEBC was identified by structural neuroendocrine characteristics resembling those of gastrointestinal/pulmonary NETs, exhibiting a neuroendocrine marker in a minimum of 50% of the total number of cells [13]. The most sensitive and specific histopathological markers of neuroendocrine tissue are CgA and Syn [14]. NEBC may also occasionally show a positive result for neuron-specific enolase (NSE) [15], whereas CD56 and other immunohistochemical indicators appear to be less sensitive and precise [16].
In 2012, the WHO raised concerns about the 2003 version, suggesting that a diagnosis may be established without a certain proportion of tumor cells manifesting neuroendocrine biomarkers. The 2012 WHO classification framework subdivides breast neoplasms exhibiting neuroendocrine properties into three distinct groups based on their morphological characteristics: (a) neuroendocrine tumor, well-differentiated (carcinoid-like), (b) poorly differentiated neuroendocrine carcinoma/small-cell carcinoma, and (c) invasive carcinoma exhibiting neuroendocrine differentiation. Well-differentiated neuroendocrine tumors are typically characterized by low to moderate nuclear-level invasive tumors. These tumors consist of spindle and plasmocytoid cellular elements, sometimes exhibiting apparent cell characteristics, and resemble carcinoid tumors originating in the gastrointestinal system and pulmonary tissues. Poorly differentiated/small-cell neuroendocrine carcinomas exhibit morphological characteristics indistinguishable from those of small-cell lung cancers. They feature a high nuclear/cytoplasmic ratio, condensed chromatin, a rapid mitotic activity, and localized necrotic zones. The third group includes various morphological variants of invasive breast carcinoma characterized by neuroendocrine differentiation, primarily exemplified by the hypercellular variants of mucinous carcinoma and the invasive type of solid papillary carcinoma [17-19].
The difficulties in differentiating between NEBC and breast tumor with neuroendocrine characteristics prompted the WHO in 2019 to reclassify breast neuroendocrine neoplasms (NENs) into well-differentiated neuroendocrine tumors (NET) and poorly differentiated NEBC, encompassing both small-cell neuroendocrine carcinoma (NEC) and large-cell NEC [20]. Breast NETs were assessed using the Nottingham scoring system, which assesses the extent of glandular tube formation, nuclear mutations, and mitotic activity in the infiltrating breast tissue. Mitotic count remains the fundamental parameter in classification systems [21]. In accordance with the Nottingham scoring system, breast NENs are classified into well-differentiated tumors (G1), intermediate-differentiated tumors (G2), or poorly differentiated carcinomas (G3). Specialized breast carcinomas (BCs) expressing neuroendocrine (NE) markers, including solid papillary carcinomas (SPCs) and mucinous carcinomas (MCs), were excluded from the neuroendocrine neoplasm (NEN) classification. Table 1 provides an overview of the various classifications provided by the WHO.
|
Table 1. Update on the latest WHO classification of NEBC. |
||||
|
Year |
Terminology |
Description |
Subgroups |
Neuroendocrine markers |
|
2003 |
Neuroendocrine tumor |
Histological characteristics analogous to those found in neuroendocrine tumors of the gastrointestinal tract and lung. |
Solid neuroendocrine carcinoma |
Chromogranin (CG) |
|
Large cell carcinoma |
Synaptophysin ( SYN) |
|||
|
50% of the cell population expresses the NE marker. |
Small cell or oat cell carcinoma |
Neuron-specific enolase (NSE) |
||
|
IBC-NOS with localized NED shown by NE markers in dispersed cells was removed. |
||||
|
2012 |
Carcinoma exhibiting neuroendocrine characteristics
|
Structural features reminiscent of those exhibited by neuroendocrine neoplasms originating in the gastrointestinal and pulmonary regions. |
Highly differentiated neuroendocrine tumor |
Chromogranin |
|
Express NE marker to a higher or a lower extent. |
Poorly differentiated neuroendocrine tumor/small cell carcinoma |
Synaptophysin |
||
|
IBC-NST and specific variants with NED were introduced. |
IBC with neuroendocrine differentiation (mucinous and solid papillary carcinoma) |
Neuron-specific enolase |
||
|
2019 |
Neuroendocrine neoplasm |
Tumour with over 90% NED. |
Neuroendocrine tumor |
Chromogranin |
|
Hypercellular mucinous carcinoma and solid papillary carcinoma were not included. |
Neuroendocrine carcinoma (NEC) (Small cell NEC; Large cell NEC) |
Synaptophysin |
||
|
CD56 |
||||
|
PGP9.5 |
||||
|
NST with neuroendocrine features in invasive breast cancer |
≤90% NE historical characteristics or NE marker expression. |
- |
- |
|
|
Mixed invasive NST and NET/NEC account for 10–90%. |
||||
|
<10% of invasive NSTs reported on the focused NE pattern. |
||||
The most prevalent NEBC subtype is small-cell NEC, which comprises diffusely proliferating neoplastic cells with tiny, darker, hyperchromatic nuclei tightly packed together, scanty cytoplasm with poorly delineated boundaries, and a high nuclear/cytoplasmic ratio under a microscope [30]. There is a high mitotic count accompanied by apoptosis, and areas of necrosis may be present. Estrogen receptors (ER) and progesterone receptors (PR) are expressed in 30–50 percent of cases [31]. Although BCL2 is often described, HER2 is not. Despite the lack of evidence for TTF-1 expression in small-cell NEC, this gene can differentiate between small-cell NEC originating in the breast and those that have spread to the lungs [32-35]. Large NEC tumor cells exhibit highly pleomorphic nuclei characterized by coarse chromatin and an adequate cytoplasm. Diagnosing large-cell NEC presents challenges, particularly compared to lung or gastrointestinal cases, owing to their resemblance to high Nottingham histological grade invasive breast carcinoma of no particular type (IBC-NST) in H&E sections. Consequently, immunohistochemical staining for neuroendocrine markers may not be performed, resulting in inadequate reporting. Fewer than ten cases of large-cell NEC have been described in the literature, with diagnosis primarily based on the expression of neuroendocrine markers [36]. Information regarding Br-MiNEN is limited because most published cases, including those in the WHO classification, do not contain clinicopathological studies about this distinct entity. Consequently, the diagnostic criteria for Br-MiNENs were derived from those established for digestive MiNENs [37, 38].
The most reliable method for diagnosing NEBC is an immunohistochemical examination of neuroendocrine biomarkers [39]. The development of immunohistochemical staining techniques allows for identifying neuroendocrine characteristics in groups of breast cancers by demonstrating their positivity for CD56, Syn, CgA, and (NSE). The neuroendocrine markers with the highest sensitivity and specificity were CgA and Syn, whereas NSE and CD56 showed lower levels of both markers. [40]. Insulinoma-associated protein 1 (INSM1) has been proposed as a reliable marker for neuroendocrine differentiation, aiding in diagnosing neuroendocrine neoplasms, particularly in cases of poor differentiation [41, 42]. Although these immunomarkers have potential utility, their application remains infrequent. Instead, they are typically reserved for instances where a highly skilled pathologist identifies or suspects neuroendocrine morphology on standard H&E-stained slides.
The majority of well-differentiated neuroendocrine tumours exhibit positive ER, PR, and AR markers. Additionally, these markers are present in more than half of poorly differentiated NEBC cases. Poorly differentiated neuroendocrine carcinomas of the breast frequently exhibit TTF1 expression, a marker typically associated with the lung lineage [43]. Additionally, up to 45% of these cases demonstrate androgen receptor (AR) expression, which is often expressed together with GCDFP15. Negative results were noted for basal markers (CK5/6, CK14, and p63) along with EGFR protein [44]. CDX2 uniformly demonstrates negative expression in primary breast neuroendocrine tumors, suggesting its potential utility in distinguishing these tumors from gastrointestinal tumors [45].
NEBCs are often positive for hormone receptors (HR) and negative for human epidermal growth factor receptor 2 (HER2) [6, 46]. They can be classified as either luminal A or luminal B molecular subtypes.
Approximately half of all NEBCs have a luminal B phenotype, an immunohistochemistry-defined HR-positive tumor with a high proliferation index ( Ki67. 14%) [47]. Furthermore, there are cases of HER2-positive NEBC [48] and small-cell breast carcinoma with basal-like features [34]. Somatostatin receptors (SSTRs), a class of G-protein coupled receptors, are found in neuroendocrine tumor cells originating from pulmonary, prostatic, and gastrointestinal tissues. Additionally, these receptors are present in ductal breast carcinoma cells [49]. Five subtypes of SSTRs have been identified, ranging from SSTR1 to SSTR5. Among these, SSTR2A is the subtype most frequently expressed in breast cancer and shows the strongest association with luminal tumors [50].
A biopsy is required for a definitive diagnosis of NEBC based on morphological characteristics and neuroendocrine biomarkers [55]. Fine Needle Aspiration (FNA) is insufficient for diagnosing NEBC because of the overlapping cytological and morphological features present in other breast lumps, including invasive ductal carcinoma and intraductal papilloma [54, 56]. Consequently, performing an imaging-guided core needle biopsy accompanied by IHC staining for neuroendocrine markers is essential to achieve a conclusive diagnosis of NEBC. Since the majority of neuroendocrine tumors arise from pulmonary and gastrointestinal origins, it is necessary to rule out metastatic lesions from other primary neuroendocrine locations, as well as other differential diagnoses such as Merkel cell carcinoma or melanoma, prior to establishing a diagnosis of primary NEBC [57]. Consequently, thoracic, and abdominal computed tomography (CT) scans should be undertaken to elucidate potential primary neoplasms associated with breast metastasis. A positron emission tomography scan (PET-FDG18) offers further diagnostic insights when distinguishing between primary and secondary neuroendocrine tumors is uncertain [58, 59]. Figure 1 illustrates the diagnostic processes associated with NEBC.
The radiological findings of NEBC are poorly understood and lack specificity. Numerous studies have indicated that NEBC typically presents with the following features: a circular, elliptical, or lobular mass with non-spiculated borders, a distinctly defined high-density lesion on a mammogram, a hypoechoic solid tumor with fuzzy boundaries, exhibiting increased vascularity, and the absence of posterior echo enhancement or a cystic component on breast scans. Magnetic resonance imaging reveals uniformly low levels of signals with heterogeneous quick initial augmentation on the T1-weighted view [60, 61]. In cases where the delineation between primary and secondary neuroendocrine tumours remains elusive, researchers have put forth various practical guidelines to facilitate differential diagnosis. Specifically, a giant tumor, the lack of an in-situ component, negative progesterone or estrogen receptor status, and no axillary nodal dissemination strongly indicate a secondary lesion rather than a primary one [62, 63].
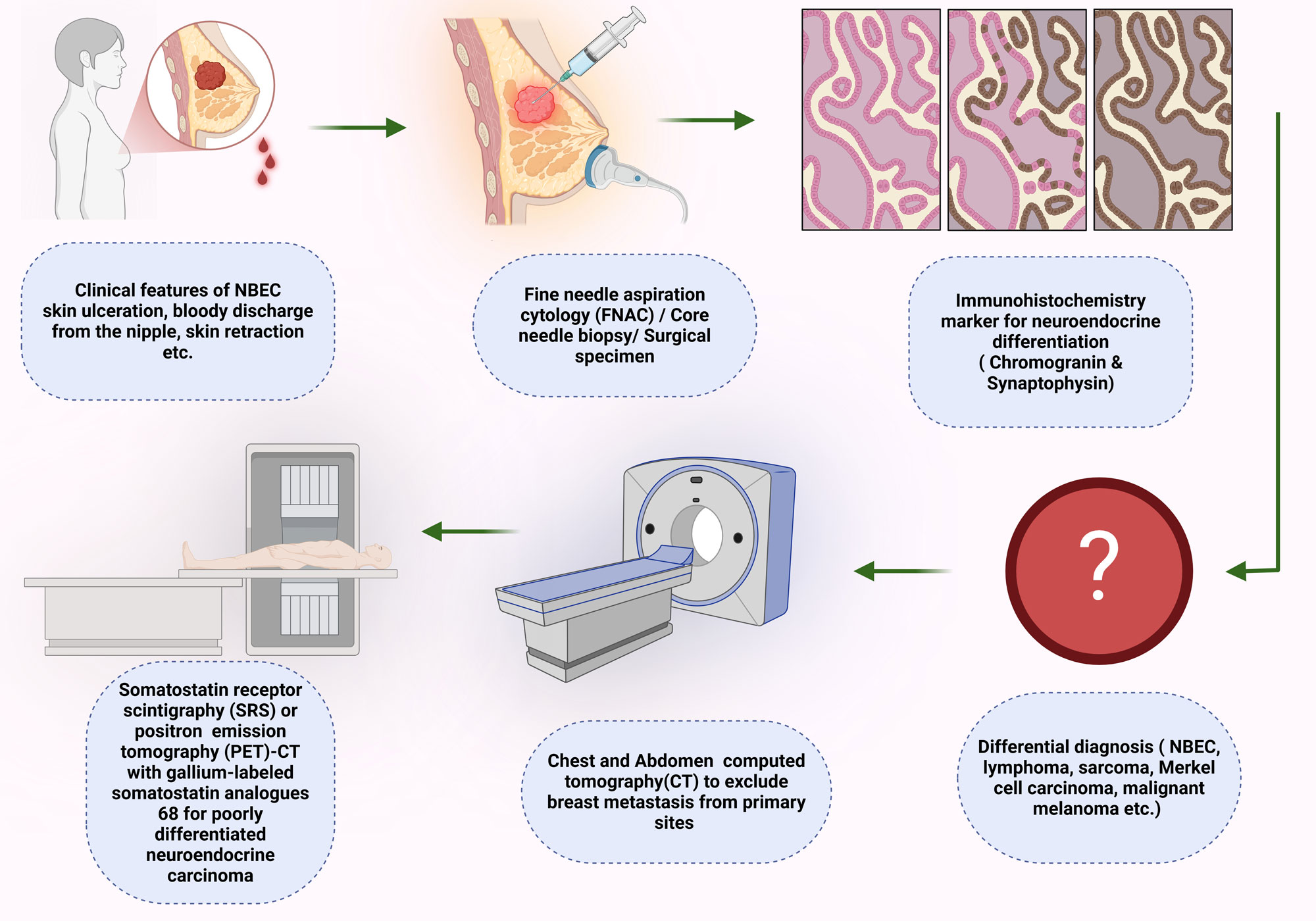 Figure 1. An illustration of the diagnostic process related to neuroendocrine breast cancer (NEBC).
Figure 1. An illustration of the diagnostic process related to neuroendocrine breast cancer (NEBC).
Radiotherapy
Currently, no dedicated studies have addressed adjuvant radiotherapy in NEBC, and its consideration should rely on the established protocols for several types of invasive breast cancer [9, 29]. Various factors, including the stage of the tumor, hormone receptor status, HER2 expression status, and the overall clinical condition of the patient influence the approach to managing NEBC through radiotherapy [70]. This method is commonly utilized after lumpectomy to reduce the chance of local recurrence and post-mastectomy when high-risk traits are identified, including significant tumour size, positive margins, or lymphatic node involvement [71, 72]. Hare et al. indicated that radiation therapy offers no advantage in small cell carcinoma of the breast [30].
Chemotherapy
Chemotherapy is an essential aspect of NEBC treatment, especially for high-grade tumors, advanced-stage disease, or metastatic dissemination. Currently, there is insufficient proof to determine the most efficient chemotherapeutic regimen. The histopathological features of NEBC may serve as a basis for the selection of appropriate chemotherapeutic agents. Typically, poorly differentiated small-cell NEC or large-cell NEC are managed using platinum and etoposide regimens [73, 74]. Other forms of NEBC are treated with taxane-based or anthracycline-based [75]. The available evidence concerning the management of NEBC through neoadjuvant chemotherapy is lacking. Sanguinetti et al. examined an aggressive NEBC by implementing neoadjuvant therapy with carboplatin and etoposide, leading to a stable condition [76, 77]. According to Wei et al., a patient with NEBC showed a significant improvement after four rounds of chemotherapy using TEC (docetaxel, epirubicin, and cyclophosphamide), which led to a substantial drop in the Ki-67 cell proliferation rate from a quarter to ten percent [75, 78]. However, a definitive suggestion could not be made because of the limited scope of knowledge.
Endocrine therapy has an apparent effect on the treatment of HR-positive breast cancer, suggesting that it could be a beneficial approach for managing NEBC. Several studies have indicated that hormonal treatment, especially when coupled with various other therapeutic strategies, is employed to treat NEBC in cases where the tumor exhibits relevant receptors [79]. Zhang et al. demonstrated that an adult NEBC patient who underwent treatment with goserelin and letrozole as neoadjuvant therapy experienced a remarkable response [80]. Neoadjuvant endocrine therapy may be employed for individuals with sizable tumors who wish to preserve the breast and oppose neoadjuvant chemotherapy. Shanks and colleagues reported a groundbreaking case involving a patient diagnosed with high-grade NEBC. This individual displayed resistance to conventional platinum-based chemotherapy and hormone therapy. However, the patient exhibited a remarkable response to a therapeutic regimen combining palbociclib, a cyclin-dependent kinase (CDK) 4/6 inhibitor, with fulvestrant [81].
The most recent molecular research has identified PIK3CA mutations in 7–33% of NEBC cases, a proportion that falls below the incidence observed in HR-positive HER2-negative IBC-NSTs [82-84]. A standard treatment approach for HR-positive, HER2-negative IBC-NSTs involves targeting the PI3K/AKT/mTOR pathway by utilizing a PI3K inhibitor (such as alpelisib) alongside an mTOR inhibitor (such as everolimus) in various research studies [85-87]. Focusing on the PI3K/AKT/mTOR signalling cascade may be a viable and encouraging approach for managing HR-positive HER2-negative NEBC.
HER2 positivity is often associated with poorly differentiated breast tumors. Targeted therapy against HER2 may be applicable in occasional cases of NEBC, whether in the adjuvant phase or during metastatic progression with HER2 overexpression. Inga et al. documented a case involving a patient who received anti-HER2 therapy in the adjuvant context for HER-2-positive primary NEBC, resulting in 9-year disease-free survival [88]. Arpine managed a case involving a patient with recurrent bone NEBC exhibiting HER2 amplification who experienced an inadequate response to treatment with trastuzumab [89].
Somatostatin receptor (SSTR) ligands play a crucial role in the biological treatment of neuroendocrine neoplasms. Immunohistochemical analyses have revealed that mammary neuroendocrine tumours express somatostatin receptor subtypes 2, 2A, 2B, 3, and 5 [90]. Global standards support the use of these analogs as the primary treatment for well-differentiated G1/2 metastatic neuroendocrine tumors. Radiolabeled SSTR-targeted imaging and peptide receptor radionuclide therapy (PRRT) has shown significant advantages in treating SSTR-expressing neuroendocrine tumors, indicating that PRRT could be an effective option for NEBC. Furthermore, The potential for metastasis in NEBCs persists for years subsequent to primary tumour treatment. Thus, it is recommended that ongoing follow-ups be conducted [91]. An overview of NEBC treatment is presented in Figure 2.
|
Table 2. Information derived from case reports and retrospective studies for treatment of NEBC. |
||||||||
|
Stage |
Subtype |
Ki67 % |
Hormonal status |
Surgical Rx |
Neoadjuvant chemotherapy |
Adjuvant chemotherapy |
Hormonal Therapy |
Ref |
|
IIIC |
Small cell |
Not reported |
ER/PR/Her- 2(-) |
Yes |
No |
Yes (CBDCA & VP-16) |
No |
[33] |
|
IIIB |
Not reported |
>20% |
ER(+) PR & Her-2(-) |
Yes |
Yes |
Yes (CDDP/VP-16) |
Yes |
[69] |
|
IIA |
Small cell |
50% |
ER/ Her-2(-) PR(+) |
Yes |
No |
Yes (CDDP/VP-16)+ FEC |
No |
[55] |
|
IIIA |
Large cell |
75% |
ER/PR/Her-2(-) |
Yes |
No |
Yes (EC) |
No |
[36] |
|
IIB |
Not reported |
90% |
ER/PR/ (+) Her-2 (-) |
Yes |
No |
Yes (FEC) |
Yes |
[70] |
|
ER,estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2; CBDCA, carboplatin; VP-16, Etoposide; CDDP, cisplatin; EC, Epirubicin/Cyclophosphamide; FEC, Fluorouracil/Epirubicin/Cyclophosphamide. |
||||||||
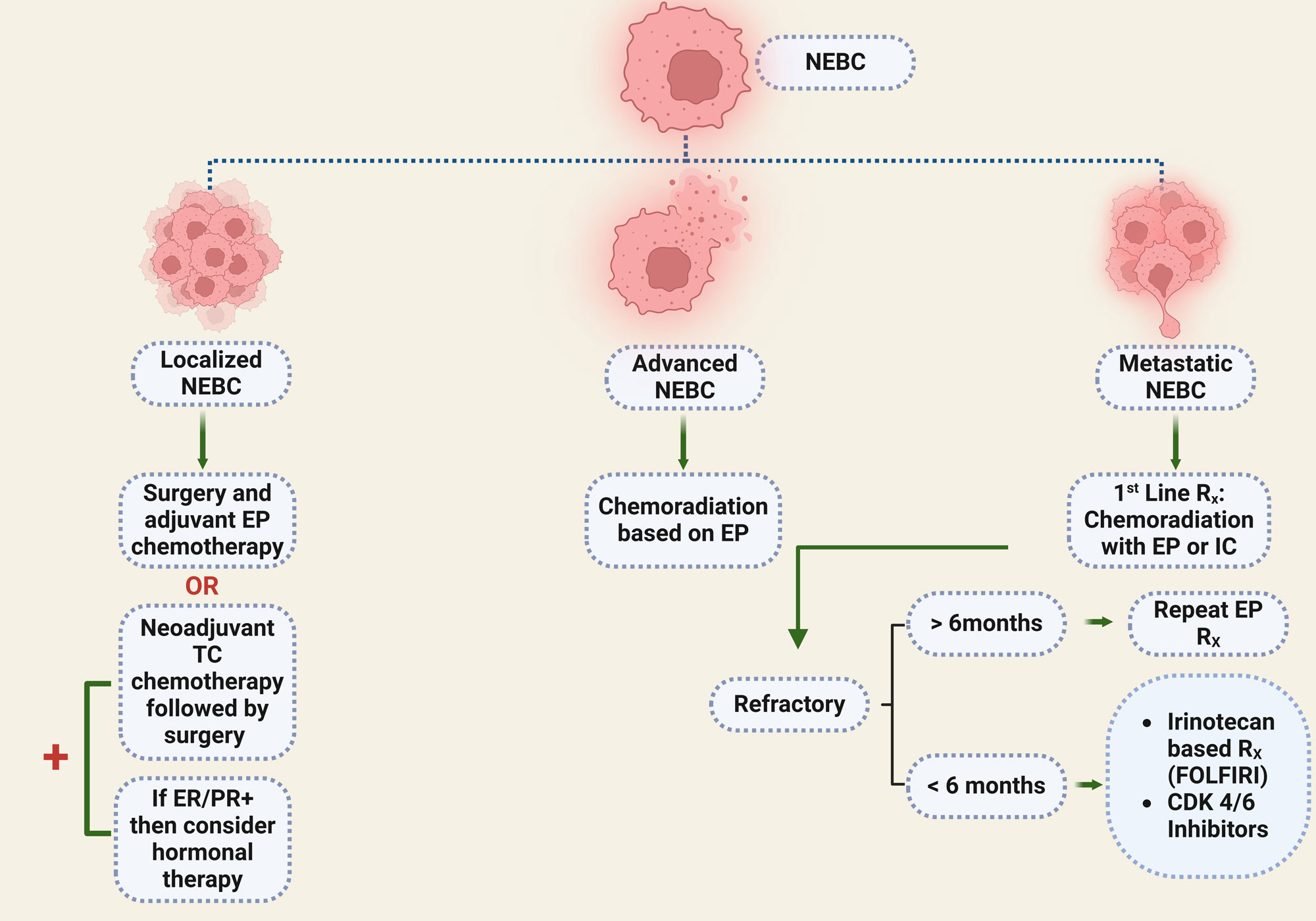 Figure 2. A protocol for the treatment of NEBC. EP, Etoposide plus platinum (cisplatin or carboplatin); TC, Taxotere and cyclophosphamide; IC, Irinotecan plus cisplatin; ER, Estrogen receptor; PR, Progesterone receptor.
Figure 2. A protocol for the treatment of NEBC. EP, Etoposide plus platinum (cisplatin or carboplatin); TC, Taxotere and cyclophosphamide; IC, Irinotecan plus cisplatin; ER, Estrogen receptor; PR, Progesterone receptor.
Additionally, cancer antigen 15-3 has been found to be considerably elevated in patients' baseline measurements and subsequently exhibited a substantial decrease following treatment, implying that CA15-3 could potentially serve as a prognostic marker [95]. Multiple investigations have demonstrated that patients presenting with tumours exceeding 20 mm in size, late-stage disease, Ki67 expression above 14%, and negative hormone receptor status are correlated with diminished OS [96]. When discussing the impact of therapeutic approaches on outcomes in NEBC, patients who did not undergo surgical intervention experienced unfavorable DSS and OS. In contrast, those treated with chemotherapy demonstrated improved DSS and OS in neuroendocrine neoplasms. According to Wei et al., radiation therapy and endocrine therapy tend to improve survival rates compared with traditional chemotherapy [97]. However, because of the small sample size and short follow-up period, none of the therapies achieved significant results in their analysis.
None.
Availability of data and materials
Data and materials are available on request from the authors.
Ethical policy
Not applicable.
Author contributions
MSA contributed to draft, critical revision of the article, figure production and submitted the final version online.
Competing interests
I declare that there is no conflict of interest regarding the publication of this document.
Funding
None.
- DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL: Breast cancer statistics, 2019. CA Cancer J Clin 2019, 69(6): 438-451.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021, 71(3): 209-249.
- Burguin A, Diorio C, Durocher F: Breast cancer treatments: updates and new challenges. J Pers Med 2021, 11(8): 808.
- Singh S, Aggarwal G, Kataria SP, Kalra R, Duhan A, Sen R: Primary neuroendocrine carcinoma of breast. J Cytol 2011, 28(2): 91-92.
- Van Der Zwan JM, Trama A, Otter R, Larrañaga N, Tavilla A, Marcos-Gragera R, Dei Tos AP, Baudin E, Poston G, Links T, et al: Rare neuroendocrine tumours: results of the surveillance of rare cancers in Europe project. Eur J Cancer 2013, 49(11): 2565-2578.
- Lavigne M, Menet E, Tille JC, Lae M, Fuhrmann L, Bonneau C, Deniziaut G, Melaabi S, Ng CC, Marchiò C, et al: Comprehensive clinical and molecular analyses of neuroendocrine carcinomas of the breast. Mod Pathol 2018, 31(1): 68-82.
- Bogina G, Munari E, Brunelli M, Bortesi L, Marconi M, Sommaggio M, Lunardi G, Gori S, Massocco A, Pegoraro MC, et al: Neuroendocrine differentiation in breast carcinoma: clinicopathological features and outcome. Histopathology 2016, 68(3): 422-432.
- Vranic S, Palazzo J, Sanati S, Florento E, Contreras E, Xiu J, Swensen J, Gatalica Z: Potential novel therapy targets in neuroendocrine carcinomas of the breast. Clin Breast Cancer, 2019, 19(2): 131-136.
- Cheymol C, Abramovici O, Do Cao C, Dumont A, Robin Y-M, El Hajbi F, Dansin E, Bonneterre J, Lauridant G: Neuroendocrine tumors of the breast: Myth or reality? A systematic review. Bull Cancer 2018, 105(4): 431-439.
- Feyrter F, Hartmann G: On the carcinoid growth form of the carcinoma mammae, especially the carcinoma solidum (gelatinosum) mammae. Frankf Z Pathol 1963, 73: 24-39.
- Cubilla AL, Woodruff JM: Primary carcinoid tumor of the breast: a report of eight patients. Am J Surg Pathol 1977, 1(4): 283-292.
- Bussolati G, Gugliotta P, Sapino A, Eusebi V, Lloyd R: Chromogranin-reactive endocrine cells in argyrophilic carcinomas (" carcinoids") and normal tissue of the breast. Am J Pathol 1985, 120(2): 186-192.
- FA T: Pathology and genetics. Tumours of the breast and female genital organs. Peritoneal tumours 2003, 4: 197-202.
- Righi L, Sapino A, Marchio C, Papotti M, Bussolati G: Neuroendocrine differentiation in breast cancer: established facts and unresolved problems. Semin Diagn Pathol 2010, 27(1): 69-76.
- Nesland JM, Holm R, Johannessen JV, Gould VE: Neurone specific enolase immunostaining in the diagnosis of breast carcinomas with neuroendocrine differentiation. Its usefulness and limitations. J Pathol 1986, 148(1): 35-43.
- Kawasaki T, Kondo T, Nakazawa T, Mochizuki K, Yamane T, Murata Si, Inoue S, Tsunoda H, Katoh R: Is CD56 a specific and reliable neuroendocrine marker for discriminating between endocrine/neuroendocrine ductal carcinoma in situ and intraductal papilloma of the breast? Pathol Int 2011, 61(1): 49-51.
- Inno A, Bogina G, Turazza M, Bortesi L, Duranti S, Massocco A, Zamboni G, Carbognin G, Alongi F, Salgarello M, et al: Neuroendocrine carcinoma of the breast: current evidence and future perspectives. Oncologist 2016, 21(1): 28-32.
- 1Park YM, Wu Y, Wei W, Yang WT: Primary neuroendocrine carcinoma of the breast: clinical, imaging, and histologic features. AJR Am J Roentgenol 2014, 203(2): W221-W230.
- Tan PH, Schnitt SJ, van de Vijver MJ, Ellis IO, Lakhani SR: Papillary and neuroendocrine breast lesions: the WHO stance. Histopathology 2015, 66(6): 761-770.
- Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al: The 2019 WHO classification of tumours of the breast. Histopathology 2020, 77(2): 181-185.
- Ozaki Y, Miura S, Oki R, Morikawa T, Uchino K: Neuroendocrine neoplasms of the breast: the latest WHO classification and review of the literature. Cancers (Basel) 2021, 14(1): 196.
- Wang J, Wei B, Albarracin CT, Hu J, Abraham SC, Wu Y: Invasive neuroendocrine carcinoma of the breast: a population-based study from the surveillance, epidemiology and end results (SEER) database. BMC cancer 2014, 14: 147.
- Makretsov N, Gilks CB, Coldman AJ, Hayes M, Huntsman D: Tissue microarray analysis of neuroendocrine differentiation and its prognostic significance in breast cancer. Hum Pathol 2003, 34(10): 1001-1008.
- Miremadi A, Pinder S, Lee A, Bell J, Paish E, Wencyk P, Elston C, Nicholson R, Blamey R, Robertson J, et al: Neuroendocrine differentiation and prognosis in breast adenocarcinoma. Histopathology 2002, 40(3): 215-222.
- Lai BSW, Tsang JY, Poon IK, Shao Y, Chan SK, Tam FK, Cheung SY, Shea KH, Tse GM: The clinical significance of neuroendocrine features in invasive breast carcinomas. The Oncologist 2020, 25(9): e1318-e1329.
- Miura K, Nasu H, Ogura H: Double neuroendocrine ductal carcinomas in situ coexisting with a background of diffuse idiopathic neuroendocrine cell hyperplasia of breast: a case report and hypothesis of neuroendocrine tumor development. Pathol Int 2012, 62(5): 331-334.
- Kawasaki T, Mochizuki K, Yamauchi H, Inoue S, Kondo T, Oishi N, Nakazawa T, Yamane T, Koshimizu Y, Tsunoda H, et al: Neuroendocrine cells associated with neuroendocrine carcinoma of the breast: nature and significance. J Clin Pathol 2012, 65(8): 699-703.
- Tang F, Wei B, Tian Z, Gilcrease MZ, Huo L, Albarracin CT, Resetkova E, Zhang H, Sahin A, Chen J, et al: Invasive mammary carcinoma with neuroendocrine differentiation: histological features and diagnostic challenges. Histopathology 2011, 59(1): 106-115.
- Tremelling A, Samuel S, Murray M: Primary small cell neuroendocrine carcinoma of the breast–A case report and review of the literature. Int J Surg Case Rep 2017, 38: 29-31.
- Hare F, Giri S, Patel JK, Hahn A, Martin MG: A population-based analysis of outcomes for small cell carcinoma of the breast by tumor stage and the use of radiation therapy. Springerplus 2015, 4(1): 1-7.
- McCullar B, Pandey M, Yaghmour G, Hare F, Patel K, Stein M, Feldman R, Chandler JC, Martin MG: Genomic landscape of small cell carcinoma of the breast contrasted to small cell carcinoma of the lung. Breast Cancer Res Treat 2016, 158(1): 195-202.
- Boutrid H, Kassem M, Tozbikian G, Morgan E, White J, Shah M, Vandeusen J, Sardesai S, Williams N, Stover DG, et al: TTF-1 positive primary small cell carcinoma of the breast: a case report and review of the literature. Front Endocrinol (Lausanne) 2020, 11: 228.
- Christie M, Chin-Lenn L, Watts MM, Tsui AE, Buchanan MR: Primary small cell carcinoma of the breast with TTF-1 and neuroendocrine marker expressing carcinoma in situ. Int J Clin Exp Pathol 2010, 3(6): 629-633.
- Erşahin Ç, Bandyopadhyay S, Bhargava R: Thyroid transcription factor-1 and “basal marker”—expressing small cell carcinoma of the breast. Int J Surg Pathol 2009, 17(5): 368-372.
- Ni YB, Tsang JY, Shao MM, Chan SK, Tong J, To KF, Tse GM: TTF‐1 expression in breast carcinoma: an unusual but real phenomenon. Histopathology 2014, 64(4): 504-511.
- Kawasaki T, Hasebe T, Oiwa M, Sugiyama K, Muramatsu C, Ueda S, Osaki A, Ichikawa J, Teramoto N, Hoshida Y: Invasive carcinoma with neuroendocrine differentiation of the breast showing triple negative, large and basal cell-like features. Pathol Int 2019, 69(8): 502-504.
- Laffi A, Fazio N, Rubino M, Spada F: Mixed Neuroendocrine and Non-neuroendocrine Neoplasms (Mi NEN). Neuroendocrine Neoplasia Manag New Approaches Diagn Treat 2021: 269-282.
- Metovic J, Cascardi E, Uccella S, Maragliano R, Querzoli G, Osella-Abate S, Pittaro A, La Rosa S, Bogina G, Cassoni P, et al: Neuroendocrine neoplasms of the breast: diagnostic agreement and impact on outcome. Virchows Arch 2022, 481(6): 839-846.
- Rakha E, Reis-Filho J, Sasano H, Wu Y: Neuroendocrine neoplasms: introduction. WHO Classif Tumours Breast Tumours 2019, 2: 155.
- Juhlin CC, Zedenius J, Höög A: Clinical routine application of the second-generation neuroendocrine markers ISL1, INSM1, and secretagogin in neuroendocrine neoplasia: staining outcomes and potential clues for determining tumor origin. Endocr Pathol 2020, 31(4): 401-410.
- Razvi H, Tsang JY, Poon IK, Chan SK, Cheung SY, Shea KH, Gary MT: INSM1 is a novel prognostic neuroendocrine marker for luminal B breast cancer. Pathology 2021, 53(2): 170-178.
- Metovic J, Castellano I, Marinelli E, Osella-Abate S, Sapino A, Cassoni P, Papotti M: INSM1 expression in breast neoplasms with neuroedocrine features. Endocr Pathol 2021, 32(4): 452-460.
- Nonaka D, Tang Y, Chiriboga L, Rivera M, Ghossein R: Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol 2008, 21(2): 192-200.
- Li X, Zhao L, Chen C, Nie J, Jiao B: Can EGFR be a therapeutic target in breast cancer? Biochim Biophys Acta Rev Cancer 2022, 1877(5): 188789.
- Wang H, Ren Y, Qian C, Liu J, Li G, Li Z: Over-expression of CDX2 alleviates breast cancer by up-regulating microRNA let-7b and inhibiting COL11A1 expression. Cancer Cell Int 2020, 20(1): 1-12.
- Weigelt B, Horlings H, Kreike B, Hayes M, Hauptmann M, Wessels L, De Jong D, Van de Vijver M, Veer LVt, Peterse J: Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol 2008, 216(2): 141-150.
- Yanagawa M, Ikemot K, Kawauchi S, Furuya T, Yamamoto S, Oka M, Oga A, Nagashima Y, Sasaki K: Luminal A and luminal B (HER2 negative) subtypes of breast cancer consist of a mixture of tumors with different genotype. BMC Res Notes 2012, 5: 376.
- Ozdemir O, Zenge B, Yildiz Y, Saray S, Alacacioglu A, Tasli F, Erdi ZC, Oflazoglu U, Taskaynatan H, Salman T, et al: Neuroendocrine Differentiated Breast Cancer Cases: A Retrospective Analysis and Literature Review. Sisli Etfal Hastan Tip Bul 2021, 55(4): 503-509.
- Terlević R, Balja MP, Tomas D, Skenderi F, Krušlin B, Vranic S, Demirović A: Somatostatin receptor SSTR2A and SSTR5 expression in neuroendocrine breast cancer. Ann Diagn Pathol 2019, 38: 62-66.
- Irelli A, Sirufo MM, Morelli L, D’Ugo C, Ginaldi L, De Martinis M: Neuroendocrine cancer of the breast: a rare entity. J Clin Med 2020, 9(5): 1452.
- Rovera F, Lavazza M, La Rosa S, Fachinetti A, Chiappa C, Marelli M, Sessa F, Giardina G, Gueli R, Dionigi G, et al: Neuroendocrine breast cancer: retrospective analysis of 96 patients and review of literature. Int J Surg 2013, 11: S79-S83.
- Lopez-Bonet E, Alonso-Ruano M, Barraza G, Vazquez-Martin A, Bernado L, Menendez JA: Solid neuroendocrine breast carcinomas: incidence, clinico-pathological features and immunohistochemical profiling. Oncol Rep 2008, 20(6): 1369-1374.
- Salemis NS: Primary neuroendocrine carcinoma of the breast: a rare presentation and review of the literature. Intractable Rare Dis Res 2020, 9(4): 233-246.
- Alqaisi S, Rahman A, Barnes M, LiPera W, Kaell AT: Routine screening mammogram leading to the incidental diagnosis of a metastatic neuroendocrine breast cancer (NEBC) from an unrecognized asymptomatic small bowel neuroendocrine tumor. Cureus 2022, 14(3): e23302.
- Abou Dalle I, Abbas J, Boulos F, Salem Z, Assi HI: Primary small cell carcinoma of the breast: a case report. J J Med Case Rep 2017, 11(1): 290.
- Kelten Talu C, Savli TC, Huq GE, Leblebici C: Histopathological and clinical differences between primary breast carcinomas with neuroendocrine features and primary breast carcinomas mimicking neuroendocrine features. Int J Surg Pathol 2019, 27(7): 744-752.
- Albright EL, Keeney ME, Bashir A, Weigel RJ: Poorly differentiated neuroendocrine carcinoma of the breast with Merkel cell features. Breast J 2018, 24(4): 644-647.
- Arslan E, Cermik TF, Trabulus FDC, Talu ECK, Başaran Ş: Diagnostic impact of 18F-FDG PET/CT on the management of rare breast carcinomas: Apocrine and neuroendocrine carcinomas. Rev Esp Med Nucl Imagen Mol (Engl Ed) 2019, 38(3): 147-153.
- Sundin A, Arnold R, Baudin E, Cwikla JB, Eriksson B, Fanti S, Fazio N, Giammarile F, Hicks RJ, Kjaer A, et al: ENETS consensus guidelines for the standards of care in neuroendocrine tumors: radiological, nuclear medicine and hybrid imaging. Neuroendocrinology 2017, 105(3): 212-244.
- Chen J, Cheng Y, You C, Zhang Y, Ye J, Ye X: Primary Neuroendocrine Carcinoma of the Breast: Mammographic, Ultrasound, and Magnetic Resonance Imaging Findings. J Med Imaging Health Inform 2020, 10(2): 279-284.
- Gharaibeh MM, Elobeid EA, El-Heis MA, Bataineh LA, Alorjani MS: Radiological and histological correlation in small cell neuroendocrine carcinoma of the breast: a case report. Am J Case Rep 2021, 22: e932274.
- Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR, Chavez-MacGregor M, Perlmutter J, et al: Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol 2020, 38(12): 1346-1366.
- Delvallée J, Etienne C, Arbion F, Vildé A, Body G, Ouldamer L: Negative estrogen receptors and positive progesterone receptors breast cancers. J Gynecol Obstet Hum Reprod 2021, 50(2): 101928.
- Tsai TH, Hsieh PP, Hong YC, Yeh CH, Yu LH, Yu MS: Metastatic primary neuroendocrine carcinoma of the breast (NECB). J Cancer Res Pract 2018, 5(1): 38-42.
- Adams R, Dyson P, Barthelmes L: Neuroendocrine breast tumours: breast cancer or neuroendocrine cancer presenting in the breast? Breast 2014, 23(2): 120-127.
- Uccella S: The classification of neuroendocrine neoplasms of the breast and its clinical relevance. Virchows Arch 2022, 481(1): 3-12.
- Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, Harbeck N, Lopez BA, Barrios C, Bergh J, et al: 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol 2019, 29(8): 1634-1657.
- Richter-Ehrenstein C, Arndt J, Buckendahl A-C, Eucker J, Weichert W, Kasajima A, Schneider A, Noske A: Solid neuroendocrine carcinomas of the breast: metastases or primary tumors? Breast Cancer Res Treat 2010, 124(2): 413-417.
- Angarita FA, Rodríguez JL, Meek E, Sánchez JO, Tawil M, Torregrosa L: Locally-advanced primary neuroendocrine carcinoma of the breast: case report and review of the literature. World J Surg Oncol 2013, 11: 128.
- Loap P, Laki F, Beuzeboc P, Fourquet A, Kirova Y: Long-Term Survival and Toxicity in Patients Treated With Radiotherapy for Neuroendocrine Breast Cancer: A Single-Center Experience. Int J Radiat Oncol Biol Phys 2021, 111(3): e219-e220.
- Singh N, Das P, Singh DK, Zaidi A: Radio-pathological characteristics of primary neuroendocrine breast carcinoma: Series of 4 cases. Radiol Case Rep 2024, 19(12): 5696-5707.
- Amsiguine N, Essaber H, Elbakkari A, Allioui S, Jerguigue H, Omor Y, Latib R: Neuroendocrine breast carcinoma: The importance of the correlation between histological and radiological findings. Radiol Case Rep 2024, 19(1): 489-492.
- Bongiovanni A, Liverani C, Foca F, Fausti V, Di Menna G, Mercatali L, De Vita A, Riva N, Calpona S, Miserocchi G, et al: Temozolomide alone or combined with capecitabine for the treatment of metastatic neuroendocrine neoplasia: A “Real-World” data analysis. Neuroendocrinology 2021, 111(9): 895-906.
- Atchison L, Hardy T, Mancl T, Quaranta BP, Madan A: Locally advanced primary small cell carcinoma of the breast: a case report and review of current evidence. Case Rep Oncol 2021, 14(2): 761-766.
- Wei X, Chen C, Xi D, Bai J, Huang W, Rong L, Wu M, Zhang G: A case of primary neuroendocrine breast carcinoma that responded to neo-adjuvant chemotherapy. Front Med 2015, 9(1): 112-116.
- Sanguinetti A, Santoprete S, Lucchini R, Triola R, Loreti F, Avenia N: A rare breast tumor: solid neuroendocrine carcinoma. Ann Ital Chir 2013, 84(1): 81-85.
- Zhang G: A case of primary neuroendocrine breast carcinoma that responded to neo-adjuvant chemotherapy. Front Med 2014, 9(1): 112-116.
- Peng L, Ma M, Zhao D, Zhao J, Sun Q, Mao F: Comparison of clinical characteristics and outcomes in primary neuroendocrine breast carcinoma versus invasive ductal carcinoma. Front Oncol 2024, 14: 1291034.
- Shin SJ, DeLellis RA, Ying L, Rosen PP: Small cell carcinoma of the breast: a clinicopathologic and immunohistochemical study of nine patients. Am J Surg Pathol 2000, 24(9): 1231-1238.
- Zhang Y, Liu C, Zheng C, Ren Q, Wang Q, Gao X, He Y, Wu J, Chen G, Li X, et al: Efficacy of neoadjuvant endocrine therapy in patients with poorly differentiated neuroendocrine carcinoma of the breast: A case report. Medicine (Baltimore) 2020, 99(43): e22652.
- Shanks A, Choi J, Karur V: Dramatic response to cyclin D–dependent kinase 4/6 inhibitor in refractory poorly differentiated neuroendocrine carcinoma of the breast. Proc (Bayl Univ Med Cent) 2018, 31(3): 352-354.
- Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, Zhang QY, Rodriguez JLM, Campone M, Hamilton E, et al: Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2−, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol 2020, 38(34): 3987-3998.
- Lavigne M, Menet E, Tille J-C, Lae M, Fuhrmann L, Bonneau C, Deniziaut G, Melaabi S, Ng CCK, Marchiò C, et al: Comprehensive clinical and molecular analyses of neuroendocrine carcinomas of the breast. Mod Pathol 2018, 31(1): 68-82.
- McCullar B, Pandey M, Yaghmour G, Hare F, Patel K, Stein M, Feldman R, Chandler JC, Martin MG: Genomic landscape of small cell carcinoma of the breast contrasted to small cell carcinoma of the lung. Breast Cancer Res Treat 2016, 158(1): 195-202.
- André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, et al: Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 2019, 380(20): 1929-1940.
- Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, et al: Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. N Engl J Med 2012, 366(6): 520-529.
- Yao JC, Pavel M, Lombard-Bohas C, Cutsem EV, Voi M, Brandt U, He W, Chen D, Capdevila J, Vries EGEd, et al: Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers From the Randomized, Phase III RADIANT-3 Study. J Clin Oncol 2016, 34(32): 3906-3913.
- Marijanović I, Kraljević M, Buhovac T, Križanac DK: Rare human epidermal growth factor receptor 2 (HER-2)-Positive neuroendocrine carcinoma of the breast: A case report with 9-year follow-up. Am J Case Rep 2020, 21: e925895-925891.
- Gevorgyan A, Bregni G, Galli G, Zanardi E, de Braud F, Di Cosimo S: HER2-positive neuroendocrine breast cancer: case report and review of literature. Breast Care (Basel) 2016, 11(6): 424-426.
- Denkert C, Lebeau A, Schildhaus HU, Jackisch C, Rüschoff J: New treatment options for metastatic HER2-low breast cancer. Pathologie (Heidelb) 2023, 44(2): 53-60.
- Valente I, Tringali G, Martella EM, Pallavera L, D'Aloia C: Primary neuroendocrine carcinoma of the breast: a case report of liver and lymph node metastases after eight years from diagnosis. Breast J 2020, 26(3): 505-507.
- Tian Z, Wei B, Tang F, Wei W, Gilcrease MZ, Huo L, Albarracin CT, Resetkova E, Middleton L, Sahin A, et al: Prognostic significance of tumor grading and staging in mammary carcinomas with neuroendocrine differentiation. Hum Pathol 2011, 42(8): 1169-1177.
- Kwon SY, Bae YK, Gu MJ, Choi JE, Kang SH, Lee SJ, Kim A, Jung HR, Kang SH, Oh HK, et al: Neuroendocrine differentiation correlates with hormone receptor expression and decreased survival in patients with invasive breast carcinoma. Histopathology 2014, 64(5): 647-659.
- Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, O’Shaughnessy J, Moroose RL, Santin AD, Abramson VG, et al: Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med 2019, 380(8): 741-751.
- Liu Q, Zhang J, Kulkarni HR, Baum RP: 177Lu-DOTATOC peptide receptor radionuclide therapy in a patient with neuroendocrine breast carcinoma and breast invasive ductal carcinoma. Clin Nucl Med 2020, 45(5): e232-e235.
- Zhang Y, Chen Z, Bao Y, Du Z, Li Q, Zhao Y, Tang F: Invasive neuroendocrine carcinoma of the breast: a prognostic research of 107 Chinese patients. Neoplasma 2013, 60(2): 215-222.
- Wei B, Ding T, Xing Y, Wei W, Tian Z, Tang F, Abraham S, Nayeemuddin K, Hunt K, Wu Y: Invasive neuroendocrine carcinoma of the breast: a distinctive subtype of aggressive mammary carcinoma. Cancer 2010, 116(19): 4463-4473.
Asia-Pacific Journal of Oncology
print ISSN: 2708-7980, online ISSN: 2708-7999
 Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript