Review Article | Open Access
Nanogold: a versatile therapeutic agent in oncology
Mater H. Mahnashia1, Bander A. Alyami1, Yahya S. Alqahtani1, Qipeng Yuan2, Arif Ullah Khan21Department of Pharmaceutical Chemistry, College of Pharmacy, Najran University, Najran, Saudi Arabia.
2Beijing Advaced Innovation Centre for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, PR China.
Correspondence: Arif Ullah Khan (Beijing Advaced Innovation Centre for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, PR China; E-mail: khanbuct@gmail.com).
Asia-Pacific Journal of Oncology 2022, 3: 16-21. https://doi.org/10.32948/ajo.2022.12.31
Received: 30 Dec 2022 | Accepted: 31 Dec 2022 | Published online: 31 Dec 2022
Key words gold nanoparticles, photothermal therapy, drug delivery, nucliec acid delivery
The unique optical activity of gold nanoparticles developed them ideal nanomaterials in biosensing, photothermal and imaging agents for medical diagnosis which is comparatively uncommon for the other inorganic nanomaterials. The high surface to volume ratio and large surface activity bestow the quality of funtionalization and large loading amounts. various molecules, including drugs, nucleic acids (DNA or RNA), proteins or peptides, antibodies, targeting ligands, and other molecules can directly or indirectly conjugate and interact with AuNPs (Figure 1) [12]. The blending capacity and miscellany highly enhance their biological properties and widen the range of their potential anticancer properties. Besides, AuNPs have been found to be comparatively stable in physiological medium because of the modification of amphiphilic materials, [13] and biocompatible, nontoxic due to inert nature of metallic gold. All of these properties have rendered AuNPs ever more popular nano-vectors in oncology. This review focuses on various widely utilized AuNPs applications in cancer treatment and diagnostics, including drug and nucleic acid delivery, photodynamic therapy (PDT), photothermal therapy (PTT), and X-ray computed tomography (CT) imaging, among others.
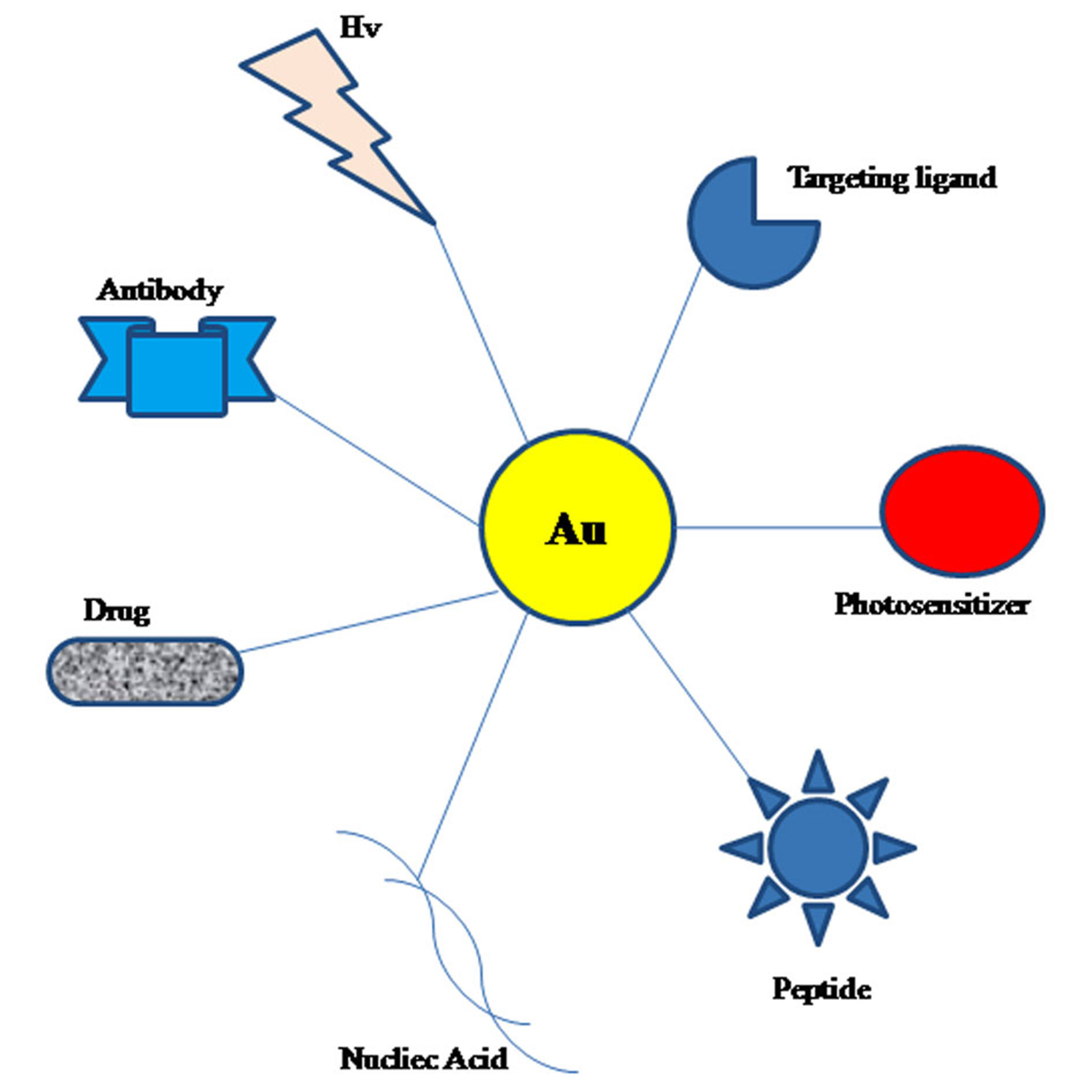 Figure 1. Different Au based nanocomplexes for efficient biological activities and diverse medical applications [25]. Abbreviation: Hv, irradiation with light.
Figure 1. Different Au based nanocomplexes for efficient biological activities and diverse medical applications [25]. Abbreviation: Hv, irradiation with light.
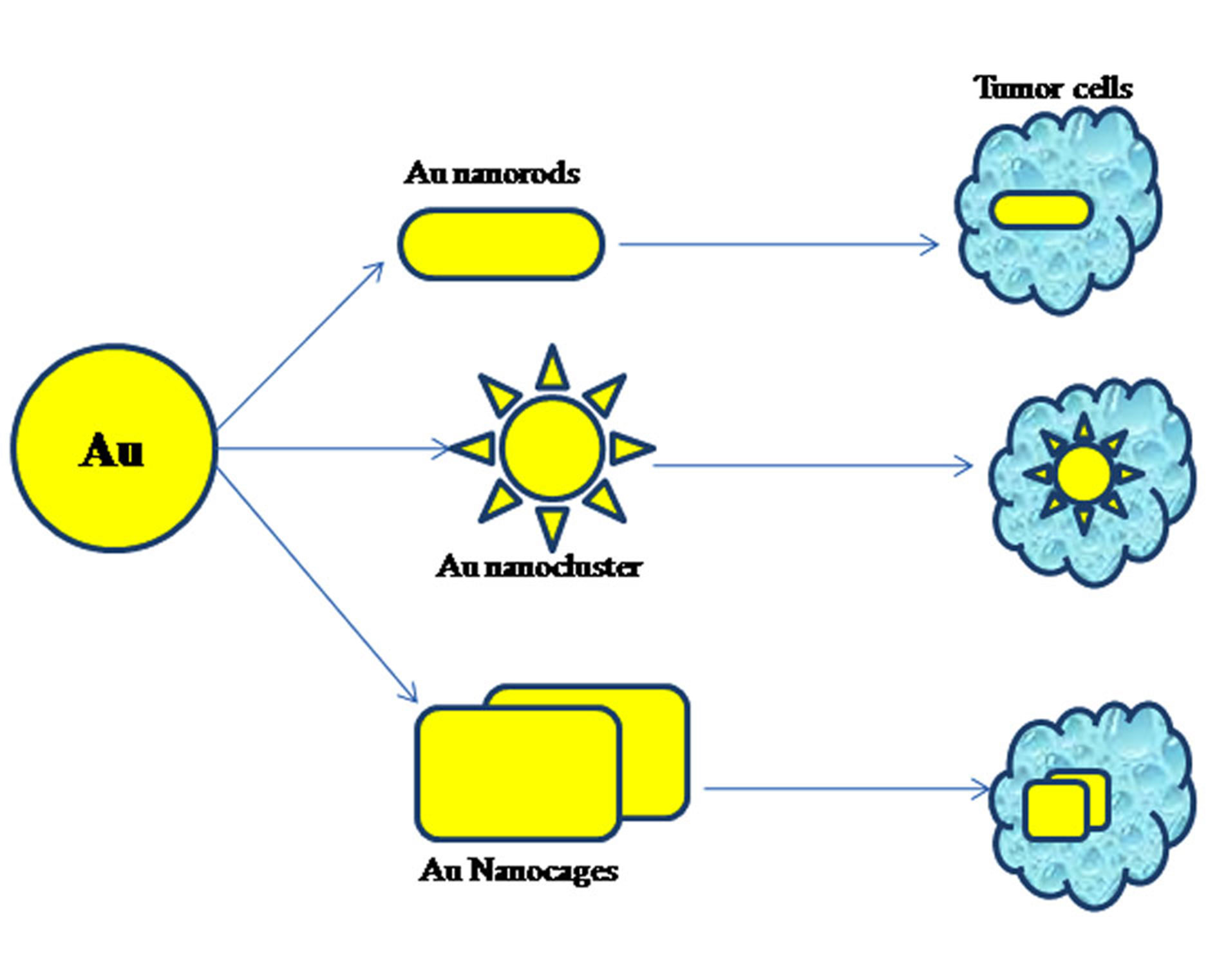 Figure 2. Different morphologies of AuNPs in cancer therapy [25].
Figure 2. Different morphologies of AuNPs in cancer therapy [25].
AuNPs surface was fabricated with tumor necrosis factor (TNF) by them to deliver TNF to the tumor tissue grown in mice. Au-TNF complex was found to have greater tumor accumulation as well as shown lower toxicity to normal cells than TNF [21].
After that AuNPs was explored deeply as a drug delivery vehicle. Many studies have reported AuNPs as drug delivery vehicle for different anticancer/antitumor medicines (Table 1), which include compounds synthesized and derived from plants [23], peptides [24] and coordination compounds [26]. These antitumor molecules have cytotoxic or regulating effects on cancer cells but some drawbacks such as low solubility, short half-life, the development of drug resistance and weak tumor selectivity limit their practical applications. One of the effective approaches is to conjugate the anticancer molecules to nanoparticles, particularly AuNPs with a “hard” core.
One of the most frequently used drugs as anticancer agent is Doxorubicin (DOX) but it is found that it induce high drug resistance in tumor tissue. In some studies, DOX could bind with stabilizer-modified AuNPs via either covalent or non-covalent interactions [30].
Different assays recommended that conjugation favored the intracellular accumulation of the DOX in drug-resistant cancer cells, indicating the chance of bypassing drug resistance in the case of conjugation.
Different internalization mechanisms could be involved in the mechanism by which drug resistance could be avoided by nanoparticle-mediated conjugation. The internalization mechanism of free DOX is different compared with the conjugated DOX that enter cells by endocytosis approach, avoiding P glycoprotein related drug resistance, as it was suggested by Wojcik et al 5-fluorouracil (5-FU) is another powerful antineoplastic drug, whose highly polar nature limits its topical use in the treatment of skin cancer. Delivery of 5-FU by cetyltrimethylammonium bromide (CTAB)-stabilized AuNPs could gain about 2-fold higher skin permeability compared with the free 5-FU formulation and achieve 6.8- and 18.4-fold lower tumor volume compared with the negative group [31].
It showed that linking hydrophilic drugs to AuNPs can help to enhance in the skin permeability and subsequent drug efficiency against skin cancer. This may have something to do with the use of stabilizer CTAB with positive charge [32]. It should be kept in mind that stabilizers or spacers seem not to be essential in the structure of the conjugated materials. Most of the drugs having carboxylic groups i.e. methotrexate (MTX) can directly link with AuNPs [33]. Free MTX showed low anticancancer activity than a very low loaded conjugate of MTX_AuNPs at an equal dose [33]. Which clearly indicate that conjugation of MTX with AuNPs significantly increase the anticancer activity of MTX.
|
Table 1. Different AuNPs complexes and their anticancer activities. |
||||||
|
Anticancer drug |
Modifying compound |
Nanom-aterial |
Cell line |
outcomes |
Mode of activity |
Reference |
|
DOX |
PEC |
DOX-PEC-AuNPs |
HepG-2 |
Efficient activity than free DOX |
Targeted delivery of DOX to hepatocarcinoma cells |
[27] |
|
DTX |
HA and GFLGC |
DTX@HA-clAuNPs |
HeLa and MCF-7 cells |
Higher cytotoxicity and tumor inhibition efficacy than free DTX under near-infrared laser irradiation |
Targeted anticancer therapy in combinationwith laser treatment |
[28] |
|
5-FU |
PEG and FA |
AuNPs-PEG5-FU-FA |
M139 and M213 cells |
Higher cytotoxic effects as compared to free 5-FU and FA |
Targeted delivery of 5-FU and targeted therapy of cholangiocarcinoma cells |
[29] |
|
LIN |
CALNN and GSH |
LIN-AuNPsCALNN |
MCF-7cells |
Higher antioxidant activity and anticancer activity as compared to Linalool and AuNPs alone |
Human breast cancer therapy |
[23] |
|
K |
- |
K-AuNPs |
MCF-7cells |
Higher cell apoptosis, antiproliferative ability and inhibition of angiogenesis compared to pure kaempferol |
Human breast cancer therapy |
[22] |
For example, Tunc et al embedded morpholino antisense oligonucleotides into a DNA-tile-AuNPs structure for treatment of breast cancer. They found that the DNA-tile-AuNPs structure delivered morpholinos and silenced the expression of HER2 and ERa gene in breast cancer cells more effectively than the liposome-based system [38]. Besides, due to the photothermal effect of AuNPs, the conjugate has the ability to become a dual functional delivery nanoplatform that achieves simultaneously gene silencing and photothermal therapy [39]. The complex still has a good photothermal effect even after nucleic acid functionalization. The composite significantly inhibited tumor growth without overt side effects for major organs after laser exposure [40]. Furthermore, AuNPs can load simultaneously gene and chemotherapy drugs to achieve a synergistic effect. Huang et al prepared a multifunctional nanoplatform based on AuNPs, which co-delivered DOX and microRNA-122, hence achieved triple therapy (gene therapy photothermal therapy and chemotherapy). With the aid of polyethylene glycol (PEG) and HA, this delivery system could selectively target hepatoma carcinoma cells without toxicity to the main organs and showed a better antitumor effect than any single therapy [41]. The release of DNA from the gold nanocomplex can be triggered by exogenous light. Upon laser irradiation, the heat generated by AuNPs through the photothermal effect is transmitted to the ambient DNA molecules. When the temperature reaches the threshold, the chemical linkages break, thus leading to DNA release [42].
Interestingly, the specific DNA release mechanisms induced by continuous wave (CW) versus pulsed lasers are different. Upon CW laser irradiation, high temperature results in dehybridization between double-stranded DNA (dsDNA) and release of nonthiolated ssDNA, while upon pulsed laser illumination, the entire DNA molecules are liberated through Au-S bond cleavage [43]. The incongruity in release mechanism makes cell mortality rate different. In a work, an anticancer drug docetaxel (DTX) was inset into complementary dsDNA that was first attached to gold nanoshells (silica core) through the Au-thiol bond for the treatment of breast cancer. The CW laser-induced DTX release caused a significant increase in breast cancer cell death, while the pulsed laser-induced drug release resulted in unobvious cell death [44]. Accordingly, AuNPs can be used as a promising genetic drug delivery vector, achieving multifunctional anti-cancer therapy.
The results may be related to urinary elimination and excretion since particles smaller than 5.5 nm can be removed rapidly and efficiently through urinary system from the body [49]. Particle shape is equally thought to be an important factor in affecting AuNP toxicity. Comparative toxicity analysis among various shaped AuNPs has already been established. Nevertheless, opinions differ in the shape effect of nanoparticles on cells. In the view of Patibandla et al, AuNRs have more deleterious effects on zebrafish than spherical AuNPs [50]. They attributed the toxicity of AuNRs to CTAB coating, which is an essential but toxic surfactant for the synthesis of AuNRs [51]. Thus, the toxicity of AuNRs can be improved by coating them with alternative biocompatible materials, such as phosphatidylcholine and PEG1 [52], underlining the impact of surface coating materials on toxicity. However, Tarantola et al pointed out that spherical AuNPs aremore toxic than rod-shaped particles due to the larger surface area ratio of spherical particles and thus higher intracellular gold content [53]. In other studies, it was observed that non-spherical (star/flower-shaped) AuNPs had relatively stronger toxicity than spherical AuNPs [54]. They attributed this outcome to the larger specific surface area presented by non-spherical AuNPs than spherical AuNPs. Higher is the internalization, more is the harmful substances carried into cells and severer is the cell damage. However, in another study, spherical and rod-shaped nanoparticles were observed to be more toxic than star-, flower- and prismshaped AuNPs [55].
The authors are thankful to Deanship of Scientific Research at Najran University under the code NU/MID/17/043 for their assistance.
Ethical policy
Not applicable.
Author contributions
Mater H. Mahnashia and Bander A. Alyami wrote the paper, Yahya S. Alqahtani and Qipeng Yuan manged the references and Arif Ullah Khan edited the manuscript.
Competing interests
The author declares that there are no conflicts of interest.
Funding
Not applicable.
- ASTM E: 2456-06 “Terminology for Nanotechnology.”. ASTM international 2006, 2.
- Nel A, Xia T, Mädler L, Li N: Toxic potential of materials at the nanolevel. Science 2006, 311(5761): 622-627.
- Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, Catsicas S, Schwaller B, Forró L: Cellular toxicity of carbon-based nanomaterials. Nano Lett 2006, 6(6): 1121-1125.
- Chang E, Thekkek N, Yu WW, Colvin VL, Drezek R: Evaluation of quantum dot cytotoxicity based on intracellular uptake. Small 2006, 2(12): 1412-1417.
- Daniel MC, Astruc D: Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 2004, 104(1): 293-346.
- Sztandera K, Gorzkiewicz M, Klajnert-Maculewicz B: Gold Nanoparticles in Cancer Treatment. Mol Pharm 2019, 16(1): 1-23.
- Bucharskaya A, Maslyakova G, Terentyuk G, Yakunin A, Avetisyan Y, Bibikova O, Tuchina E, Khlebtsov B, Khlebtsov N, Tuchin V: Towards Effective Photothermal/Photodynamic Treatment Using Plasmonic Gold Nanoparticles. Int J Mol Sci 2016, 17(8): 1295.
- Liu Y, Crawford BM, Vo-Dinh T: Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy 2018, 10(13): 1175-1188.
- D RM, B PG, Abrahamse H: Enhancing Breast Cancer Treatment Using a Combination of Cannabidiol and Gold Nanoparticles for Photodynamic Therapy. Int J Mol Sci 2019, 20(19): 4771.
- Shrestha B, Wang L, Zhang H, Hung CY, Tang L: Gold Nanoparticles Mediated Drug-Gene Combinational Therapy for Breast Cancer Treatment. Int J Nanomedicine 2020, 15: 8109-8119.
- Mioc A, Mioc M, Ghiulai R, Voicu M, Racoviceanu R, Trandafirescu C, Dehelean C, Coricovac D, Soica C: Gold Nanoparticles as Targeted Delivery Systems and Theranostic Agents in Cancer Therapy. Curr Med Chem 2019, 26(35): 6493-6513.
- Ramalingam V: Multifunctionality of gold nanoparticles: Plausible and convincing properties. Adv Colloid Interface Sci 2019, 271: 101989.
- Kong FY, Zhang JW, Li RF, Wang ZX, Wang WJ, Wang W: Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22(9): 1445.
- Boisselier E, Astruc D: Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev 2009, 38(6): 1759-1782.
- Onaciu A, Braicu C, Zimta AA, Moldovan A, Stiufiuc R, Buse M, Ciocan C, Buduru S, Berindan-Neagoe I: Gold nanorods: from anisotropy to opportunity. An evolution update. Nanomedicine (Lond) 2019, 14(9): 1203-1226.
- Vucic S, Kiernan MC, Menon P, Huynh W, Rynders A, Ho KS, Glanzman R, Hotchkin MT: Study protocol of RESCUE-ALS: A Phase 2, randomised, double-blind, placebo-controlled study in early symptomatic amyotrophic lateral sclerosis patients to assess bioenergetic catalysis with CNM-Au8 as a mechanism to slow disease progression. BMJ Open 2021, 11(1): e041479.
- Chen F, Si P, de la Zerda A, Jokerst JV, Myung D: Gold nanoparticles to enhance ophthalmic imaging. Biomater Sci 2021, 9(2): 367-390.
- Yang J, Zhai S, Qin H, Yan H, Xing D, Hu X: NIR-controlled morphology transformation and pulsatile drug delivery based on multifunctional phototheranostic nanoparticles for photoacoustic imaging-guided photothermal-chemotherapy. Biomaterials 2018, 176: 1-12.
- Maysinger D, Gran ER, Bertorelle F, Fakhouri H, Antoine R, Kaul ES, Samhadaneh DM, Stochaj U: Gold nanoclusters elicit homeostatic perturbations in glioblastoma cells and adaptive changes of lysosomes. Theranostics 2020, 10(4): 1633-1648.
- Chinnaiyan SK, Soloman AM, Perumal RK, Gopinath A, Balaraman M: 5 Fluorouracil-loaded biosynthesised gold nanoparticles for the in vitro treatment of human pancreatic cancer cell. IET Nanobiotechnol 2019, 13(8): 824-828.
- Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L: Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv 2004, 11(3): 169-183.
- Raghavan BS, Kondath S, Anantanarayanan R, Rajaram R: Kaempferol mediated synthesis of gold nanoparticles and their cytotoxic effects on MCF-7 cancer cell line. Process Biochem 2015, 50(11): 1966-1976.
- Jabir MS, Taha AA, Sahib UI, Taqi ZJ, Al-Shammari AM, Salman AS: Novel of nano delivery system for Linalool loaded on gold nanoparticles conjugated with CALNN peptide for application in drug uptake and induction of cell death on breast cancer cell line. Mater Sci Eng C Mater Biol Appl 2019, 94: 949-964.
- Kapur A, Medina SH, Wang W, Palui G, Schneider JP, Mattoussi H: Intracellular Delivery of Gold Nanocolloids Promoted by a Chemically Conjugated Anticancer Peptide. ACS Omega 2018, 3(10): 12754-12762.
- Yang Z, Wang D, Zhang C, Liu H, Hao M, Kan S, Liu D, Liu W: The Applications of Gold Nanoparticles in the Diagnosis and Treatment of Gastrointestinal Cancer. Front Oncol 2021, 11: 819329.
- Fernandes AR, Jesus J, Martins P, Figueiredo S, Rosa D, Martins LM, Corvo ML, Carvalheiro MC, Costa PM, Baptista PV: Multifunctional gold-nanoparticles: A nanovectorization tool for the targeted delivery of novel chemotherapeutic agents. J Control Release 2017, 245: 52-61.
- Borker S, Pokharkar V: Engineering of pectin-capped gold nanoparticles for delivery of doxorubicin to hepatocarcinoma cells: an insight into mechanism of cellular uptake. Artif Cells Nanomed Biotechnol 2018, 46(sup2): 826-835.
- Gotov O, Battogtokh G, Ko YT: Docetaxel-Loaded Hyaluronic Acid-Cathepsin B-Cleavable-Peptide-Gold Nanoparticles for the Treatment of Cancer. Mol Pharm 2018, 15(10): 4668-4676.
- Ngernyuang N, Seubwai W, Daduang S, Boonsiri P, Limpaiboon T, Daduang J: Targeted delivery of 5-fluorouracil to cholangiocarcinoma cells using folic acid as a targeting agent. Mater Sci Eng C Mater Biol Appl 2016, 60: 411-415.
- Wójcik M, Lewandowski W, Król M, Pawłowski K, Mieczkowski J, Lechowski R, Zabielska K: Correction: enhancing anti-tumor efficacy of Doxorubicin by non-covalent conjugation to gold nanoparticles - in vitro studies on feline fibrosarcoma cell lines. PLoS One 2015, 10(6): e0129639.
- Safwat MA, Soliman GM, Sayed D, Attia MA: Fluorouracil-Loaded Gold Nanoparticles for the Treatment of Skin Cancer: Development, in Vitro Characterization, and in Vivo Evaluation in a Mouse Skin Cancer Xenograft Model. Mol Pharm 2018, 15(6): 2194-2205.
- Som I, Bhatia K, Yasir M: Status of surfactants as penetration enhancers in transdermal drug delivery. J Pharm Bioallied Sci 2012, 4(1): 2-9.
- Álvarez-González B, Rozalen M, Fernández-Perales M, Álvarez MA, Sánchez-Polo M: Methotrexate Gold Nanocarriers: Loading and Release Study: Its Activity in Colon and Lung Cancer Cells. Molecules 2020, 25(24): 6049.
- Han G, Martin CT, Rotello VM: Stability of gold nanoparticle-bound DNA toward biological, physical, and chemical agents. Chem Biol Drug Des 2006, 67(1): 78-82.
- Chan GG, Koch CM, Connors LH: Blood Proteomic Profiling in Inherited (ATTRm) and Acquired (ATTRwt) Forms of Transthyretin-Associated Cardiac Amyloidosis. J Proteome Res 2017, 16(4): 1659-1668.
- Riley MK, Vermerris W: Recent Advances in Nanomaterials for Gene Delivery-A Review. Nanomaterials (Basel) 2017, 7(5): 94.
- Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AK, Han MS, Mirkin CA: Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 2006, 312(5776): 1027-1030.
- Tunç CU, Öztaş DY, Uzunoğlu D, Bayrak Ö F, Çulha M: Silencing Breast Cancer Genes Using Morpholino Embedded DNA-Tile-AuNPs Nanostructures. Hum Gene Ther 2019, 30(12): 1547-1558.
- Karimi S, Fouani MH, Moshaii A, Nikkhah M, Hosseinkhani S, Sheikhnejad R: Development of Dual Functional Nucleic Acid Delivery Nanosystem for DNA Induced Silencing of Bcl-2 Oncogene. Int J Nanomedicine 2020, 15: 1693-1708.
- Liu Y, Xu M, Zhao Y, Chen X, Zhu X, Wei C, Zhao S, Liu J, Qin X: Flower-like gold nanoparticles for enhanced photothermal anticancer therapy by the delivery of pooled siRNA to inhibit heat shock stress response. J Mater Chem B 2019, 7(4): 586-597.
- Huang S, Liu Y, Xu X, Ji M, Li Y, Song C, Duan S, Hu Y: Triple therapy of hepatocellular carcinoma with microRNA-122 and doxorubicin co-loaded functionalized gold nanocages. J Mater Chem B 2018, 6(15): 2217-2229.
- Lee K, Kim T, Kim YM, Yang K, Choi I, Roh YH: Multifunctional DNA Nanogels for Aptamer-Based Targeted Delivery and Stimuli-Triggered Release of Cancer Therapeutics. Macromol Rapid Commun 2021, 42(2): e2000457.
- Goodman AM, Hogan NJ, Gottheim S, Li C, Clare SE, Halas NJ: Understanding Resonant Light-Triggered DNA Release from Plasmonic Nanoparticles. ACS Nano 2017, 11(1): 171-179.
- Goodman AM, Neumann O, Nørregaard K, Henderson L, Choi MR, Clare SE, Halas NJ: Near-infrared remotely triggered drug-release strategies for cancer treatment. Proc Natl Acad Sci U S A 2017, 114(47): 12419-12424.
- Rambanapasi C, Zeevaart JR, Buntting H, Bester C, Kotze D, Hayeshi R, Grobler A: Bioaccumulation and Subchronic Toxicity of 14 nm Gold Nanoparticles in Rats. Molecules 2016, 21(6): 763.
- Enea M, Pereira E, Silva DD, Costa J, Soares ME, de Lourdes Bastos M, Carmo H: Study of the intestinal uptake and permeability of gold nanoparticles using both in vitro and in vivo approaches. Nanotechnology 2020, 31(19): 195102.
- Lopez-Chaves C, Soto-Alvaredo J, Montes-Bayon M, Bettmer J, Llopis J, Sanchez-Gonzalez C: Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomedicine 2018, 14(1): 1-12.
- Chen YS, Hung YC, Liau I, Huang GS: Assessment of the In Vivo Toxicity of Gold Nanoparticles. Nanoscale Res Lett 2009, 4(8): 858-864.
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV: Renal clearance of quantum dots. Nat Biotechnol 2007, 25(10): 1165-1170.
- Patibandla S, Zhang Y, Tohari AM, Gu P, Reilly J, Chen Y, Shu X: Comparative analysis of the toxicity of gold nanoparticles in zebrafish. J Appl Toxicol 2018, 38(8): 1153-1161.
- Alkilany AM, Nagaria PK, Hexel CR, Shaw TJ, Murphy CJ, Wyatt MD: Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small 2009, 5(6): 701-708.
- Takahashi H, Niidome Y, Niidome T, Kaneko K, Kawasaki H, Yamada S: Modification of gold nanorods using phosphatidylcholine to reduce cytotoxicity. Langmuir 2006, 22(1): 2-5.
- Tarantola M, Pietuch A, Schneider D, Rother J, Sunnick E, Rosman C, Pierrat S, Sönnichsen C, Wegener J, Janshoff A: Toxicity of gold-nanoparticles: synergistic effects of shape and surface functionalization on micromotility of epithelial cells. Nanotoxicology 2011, 5(2): 254-268.
- Sultana S, Djaker N, Boca-Farcau S, Salerno M, Charnaux N, Astilean S, Hlawaty H, de la Chapelle ML: Comparative toxicity evaluation of flower-shaped and spherical gold nanoparticles on human endothelial cells. Nanotechnology 2015, 26(5): 055101.
- Woźniak A, Malankowska A, Nowaczyk G, Grześkowiak BF, Tuśnio K, Słomski R, Zaleska-Medynska A, Jurga S: Size and shape-dependent cytotoxicity profile of gold nanoparticles for biomedical applications. J Mater Sci Mater Med 2017, 28(6): 92.
Asia-Pacific Journal of Oncology
print ISSN: 2708-7980, online ISSN: 2708-7999
 Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript