Review | Open Access
Research progress on the mechanisms, assessment methods, and intervention strategies for glioma-related cognitive impairment
Riffat Iqbal1, Zeeshan Ashraf2
1Department of Zoology, Government College University Lahore, Pakistan.
2Department of Zoology, University of Education, Faisalabad Campus, Punjab, Pakistan.
Correspondence: Zeeshan Ashraf (Department of Zoology, University of Education, Faisalabad Campus, Punjab, Pakistan; Email: Zeeshandod123@gmail.com).
Asia-Pacific Journal of Oncology 2024, 5: 46-54. https://doi.org/10.32948/ajo.2024.09.09
Received: 27 Aug 2024 | Accepted: 07 Sep 2024 | Published online: 12 Sep 2024
Key words glioma, cognitive dysfunction, neuroinflammation, neuropsychological assessment, intervention strategies, transcranial magnetic stimulation, brain-computer interface
The mechanisms underlying glioma-related cognitive dysfunction are complex and involve multiple factors, such as the direct effects of the tumor, side effects of treatment, and long-term pathological changes in the central nervous system [7-11]. First, the growth of the tumor itself compresses critical brain regions, especially when located in the frontal, temporal, or parietal lobes, which are closely associated with cognitive function. Second, although surgical resection of the tumor can relieve pressure on brain tissue, it may also cause damage to the brain, leading to cognitive dysfunction. Additionally, while radiotherapy and chemotherapy are effective in delaying tumor progression, the damage inflicted on healthy brain tissues cannot be overlooked. Whole-brain radiotherapy can cause extensive white matter damage, reduce neural conduction efficiency, and lead to cognitive decline. Chemotherapeutic agents such as temozolomide have also been shown to cross the blood-brain barrier, exerting toxic effects on neurones and worsening cognitive dysfunction [12, 13].
Neuroinflammation is considered a significant mechanism in glioma-related cognitive dysfunction. Research indicates that Gliomas can trigger neuroinflammatory responses, leading to neuronal dysfunction and remodelling of neural networks [14-17]. Proinflammatory factors released by tumor cells and their microenvironment, such as tumor necrosis factor (TNF-α) and interleukins (IL-6), activate microglia and astrocytes, initiating widespread neuroinflammatory responses [18-21]. Chronic inflammation not only damages neurons but can also compromise the permeability of the blood-brain barrier, allowing peripheral toxic substances to invade the central nervous system, further exacerbating neural damage. Moreover, the integrity of the white matter in glioma patients is often disrupted, with the degeneration of white matter tracts potentially impairing neural signal transmission and contributing to cognitive decline.
Various tools are currently being employed to better assess the cognitive function of glioma patients; various tools are currently employed. Neuropsychological testing is one of the most commonly used methods to quantify patient performance across different cognitive domains such as attention, memory, language, and executive function to provide a comprehensive understanding of cognitive impairment [22, 23]. However, traditional neuropsychological tests are often time-consuming and subject to external factors such as the testing environment and patient mood, making it difficult to accurately reflect the true cognitive state of glioma patients. Van Oostveen WM has provided new insights into cognitive assessment [24]. Functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) are additional tools that can indirectly assess cognitive function by observing changes in functional brain areas and white matter integrity [25, 26]. These techniques offer objective data and can be used to predict cognitive recovery potential and treatment efficacy.
Recent advancements have also been made in intervention strategies for glioma-related cognitive dysfunction [27-30]. Pharmacological treatments are common interventions, with drugs such as memantine (an NMDA receptor antagonist) and donepezil (a cholinesterase inhibitor), which are widely used to improve cognitive function. However, the effectiveness of these treatments in glioma patients remains controversial, and long-term use may lead to adverse effects. In addition to pharmacological approaches, neurorehabilitation training has gained attention, particularly in individualized training programs designed to target specific cognitive deficits that have shown promise in improving cognitive performance. Emerging non-invasive technologies, such as transcranial magnetic stimulation (TMS) and brain-computer interfaces (BCI), also have the potential to restore cognitive function. TMS enhances neuronal function and brain network connectivity through external magnetic field stimulation, while BCI technology seeks to restore partial cognitive abilities by facilitating direct interaction between the brain and external devices.
In summary, glioma-related cognitive dysfunction involves multiple contributing factors, and assessment methods are becoming increasingly diverse while intervention strategies continue to advance. Future research should focus on further exploring the clinical effectiveness of these interventions and strive to improve the quality of life for glioma patients by integrating individualized assessments and treatments.
The illustration portrays the intricate landscape of Glioma-Related Cognitive Dysfunction, encompassing a spectrum of factors and interventions. At its core are the Mechanisms of Cognitive Impairment, which are influenced by Tumor impact, surgical treatment effects, and side effects of radiotherapy/chemotherapy. These elements can trigger neuroinflammation and impair neuroplasticity, subsequently affecting White Matter integrity. To assess the extent of these impairments, Cognitive Assessment Tools are employed, including Neuropsychological Tests and advanced Imaging Examinations such as fMRI and DTI. The figure also highlights various Pharmacological Interventions, specifically Memantine and Donepezil, that are employed to mitigate cognitive decline. Additionally, non-pharmacological approaches like Neurorehabilitation Training, Transcranial Magnetic Stimulation (TMS), and Brain-Computer Interface (BCI) are presented as innovative strategies to enhance cognitive function. This figure suggests the importance of personalized treatment plans and integrated Multimodal Interventions to tailor therapies according to individual patient needs. Looking forward, the caption points to Future Research Directions and the development of Clinical Guidelines, emphasizing the ongoing efforts to improve treatment outcomes and quality of life in patients with glioma-related cognitive dysfunction.
Impact of the tumor itself
The impact of gliomas on cognitive function is closely related to the tumor's location and size. Different brain regions are responsible for specific cognitive functions. For instance, the frontal lobe is primarily involved in executive functions and higher cognitive processes, while Menon V found that the temporal lobe is associated with memory and language, and the parietal lobe plays a role in spatial cognition and attention [31]. The location of the tumor significantly influenced the type and extent of cognitive impairment. Gliomas located in the frontal lobe may lead to executive function disorders, such as difficulties with decision-making, reduced planning ability, and impaired social cognition. Tumors in the temporal lobe can cause language difficulties, memory decline, aphasia, and amnesia. Patients with parietal lobe tumors often experience challenges with spatial cognition and attention, which severely affects their daily living and social interactions.
In addition to the local compression effects of the tumor, its invasive growth can also damage the structure and function of the adjacent normal brain tissue, further exacerbating cognitive impairment. Due to the high invasiveness of gliomas, they frequently spread along nerve fibers and blood vessels, causing extensive damage to the brain tissue. This damage extends beyond the tumor's growth area, affecting broader brain network structures through diffusion effects and further affecting cognitive function. Pathological changes such as local brain edema, hemorrhage, and ischemia caused by the tumor contribute to the complex and diverse manifestations of cognitive dysfunction in glioma patients.
Impact of treatment factors
Treatment methods for gliomas include surgery, radiotherapy, and chemotherapy; however, while targeting tumor cells, these treatments often cause varying degrees of damage to the central nervous system, thus affecting cognitive function.
Surgical treatment is one of the primary methods for managing gliomas, with the aim of removing as much of the tumor as possible to alleviate pressure on the brain tissue. However, surgery inevitably damages the surrounding normal brain tissue, especially when the tumor is located near the functional areas. The removal of the tumor may result in irreversible neuronal damage and further worsening of cognitive function. Additionally, Jia S found that postoperative complications, such as brain edema and inflammatory responses, can hinder cognitive recovery, making postoperative cognitive dysfunction more complex [32].
Radiotherapy, particularly whole-brain radiotherapy, is effective in controlling tumor growth but often causes irreversible damage to white matter fibers. Winter SF showed that radiotherapy can induce widespread white matter degeneration, leading to the rupture of neural fiber tracts and significant reductions in neural signal conduction efficiency [33]. Children and elderly patients are especially susceptible to severe cognitive decline following radiotherapy and experience widespread reductions in attention, memory, and language function. Moreover, Ramírez-Guerrero S reported that radiotherapy may damage the hippocampus and other regions closely related to memory, further exacerbating cognitive impairment [34].
Chemotherapy, as an adjunctive treatment for gliomas, commonly involves drugs such as temozolomide, which can cross the blood-brain barrier and directly target tumor cells in the brain [35]. However, chemotherapeutic agents may also exert toxic effects on normal neurons and glial cells. Long-term chemotherapy can lead to brain atrophy, white matter damage, and neural network dysfunctions. Cognitive impairments caused by chemotherapy, often referred to as "chemobrain," are characterized by persistent attention deficits, memory decline, and slowed information processing. These impairments may persist long after treatment and result in permanent cognitive damage in some cases.
Neuroinflammation mechanism
Neuroinflammation is a significant pathological mechanism underlying glioma-related cognitive dysfunction. The presence of the tumor triggers both local and systemic inflammatory responses, altering the microenvironment within the central nervous system and affecting neuronal function. Pro-inflammatory factors, such as tumor necrosis factor (TNF-α) and interleukins (IL-6), released within the tumor microenvironment, are considered major contributors to neuroinflammation [36, 37]. These inflammatory factors not only directly activate microglia and astrocytes within the brain but also influence the infiltration of peripheral immune cells through the blood-brain barrier, which further exacerbates the inflammatory response in the brain.
According to Borst K, microglia are the primary immune cells in the central nervous system and play a key role in neuroinflammation [38]. When activated, microglia release large quantities of inflammatory factors and free radicals, leading to neuronal damage and the disruption of neural networks. Astrocytes indirectly affect the maintenance and repair of cognitive functions by regulating neuronal metabolism and the permeability of the blood-brain barrier. However, prolonged neuroinflammatory responses can result in excessive neuronal damage and neurodegeneration, further exacerbating cognitive impairment.
White matter integrity disruption
White matter fiber tracts connect different brain regions and transmit neural signals. The integrity of the white matter is essential for maintaining normal cognitive function. However, both tumor-induced compression and side effects of treatment can cause significant white matter damage, adversely affecting cognitive function. Glioma-induced local compression leads to pressure and rupture of white matter fiber tracts, while radiotherapy and chemotherapy often result in widespread white matter degeneration and damage to fiber tracts.
White matter damage frequently results in a marked decline in neural conduction efficiency, particularly in cognitive tasks that require long-distance connections between the brain regions. Patients may experience noticeable delays in response time and difficulties in information processing. Imaging techniques, such as diffusion tensor imaging (DTI), have revealed structural disruptions and functional impairments in white matter fiber tracts in glioma patients, and these damages are significantly correlated with cognitive decline [39]. In high-load cognitive tasks, white matter damage substantially prolongs the transmission time of neural signals, leading to delayed responses, impaired executive functions, and reduced spatial cognitive abilities.
The mechanisms of glioma-related cognitive dysfunction are complex and multifaceted, involving not only direct tumor compression and invasion but also treatment-induced damage to brain tissue, persistent neuroinflammation, and the destruction of white matter structures. Together, these mechanisms contribute to the diverse and highly individualized manifestations of cognitive dysfunction in glioma patients. Understanding these mechanisms is crucial for developing more effective treatment and intervention strategies to alleviate cognitive impairments and improve patients' quality of life.
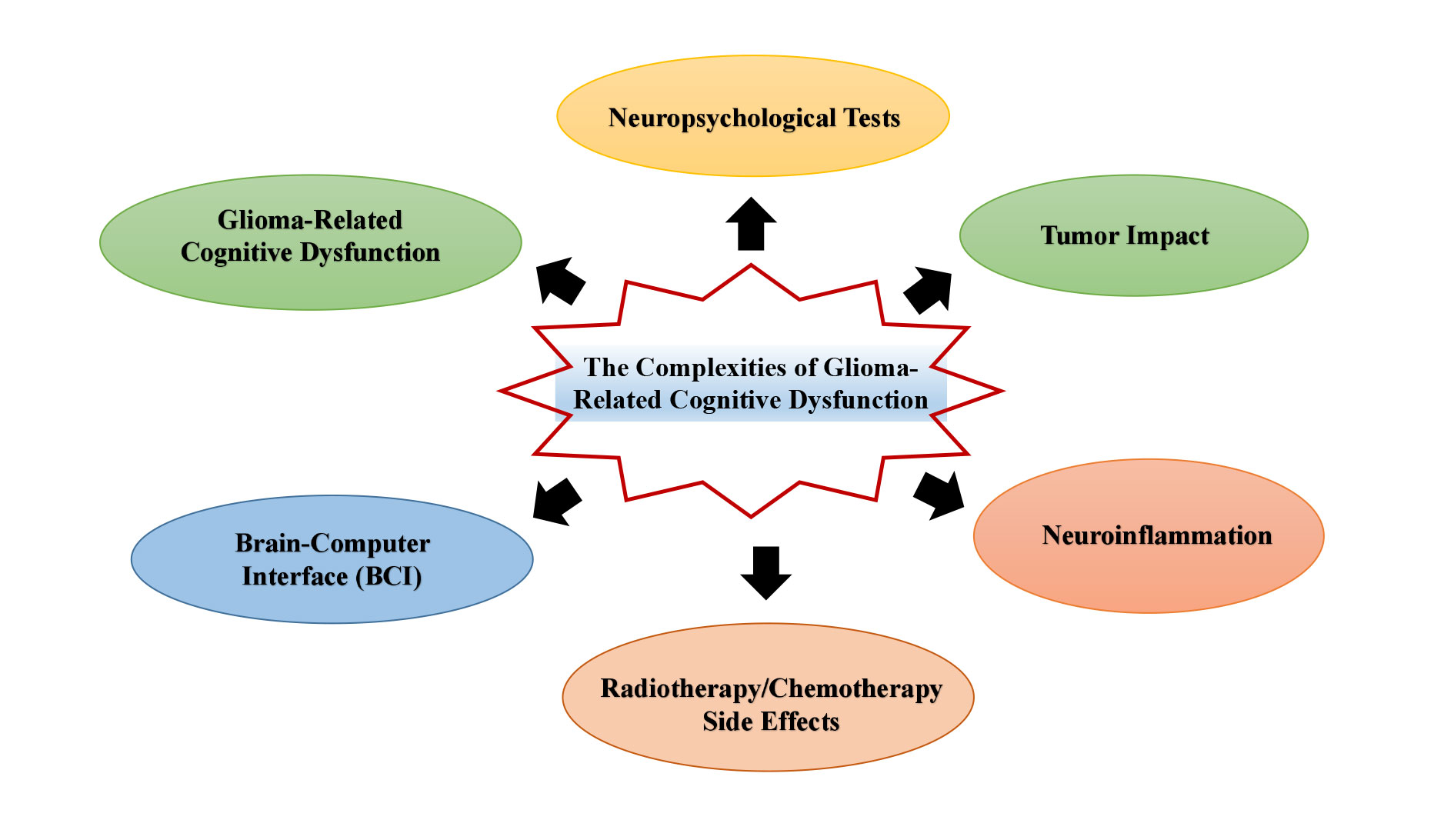 Figure 1. Exploring the complexities of glioma-related cognitive dysfunction: mechanisms, assessment, and therapie.
Figure 1. Exploring the complexities of glioma-related cognitive dysfunction: mechanisms, assessment, and therapie.
This image illustrates the complex relationship between glioma cells and cognitive dysfunction, with a particular focus on the role of inflammatory factors and the blood-brain barrier. Cognitive dysfunction, a common consequence of gliomas, encompasses a range of impairments including memory decline, attention deficits, and disruptions in executive function. Inflammatory factors released in response to glioma cells can further exacerbate these cognitive issues by inducing neuroinflammation and disrupting neuronal signaling. The blood-brain barrier, a critical interface regulating the exchange of molecules between the bloodstream and the brain, is highlighted as a key factor in both the progression of gliomas and modulation of cognitive impairments. Additionally, the concept of an 'inflammatory barrier' suggests that changes in the blood-brain barrier may be linked to the inflammatory response associated with gliomas.
Neuropsychological tests
Neuropsychological tests are traditional methods for evaluating cognitive dysfunction, primarily through quantitative tools that assess patient performance across various cognitive domains such as memory, attention, executive function, language ability, and spatial cognition. These tests are widely used in clinical practice, particularly for glioma patients, where the impact on different brain areas is localized. In such cases, neuropsychological tests can effectively identify specific cognitive impairments.
Commonly used neuropsychological test tools include (the) Mini-Mental State Examination (MMSE), which is used for rapid assessment of cognitive status, including orientation to time and place, memory, attention, and language functions. While the MMSE provides a general overview of cognitive function, it has limitations in evaluating complex higher-order cognitive functions. (b) Montreal Cognitive Assessment (MoCA): Compared to the MMSE, the MoCA is more sensitive to mild cognitive impairments and is better at assessing executive function, language, abstract thinking, and delayed memory. (c) Wechsler Memory Scale (WMS): This test specifically assesses memory function and distinguishes between short- and long-term memory impairments, making it suitable for evaluating patients with hippocampal damage. (d) Stroop Color and Word Test: A classic test for evaluating executive function, which measures selective attention, inhibitory control, and cognitive flexibility.
However, neuropsychological tests are limited. First, these tools rely on patients’ language abilities and cooperation, and the results may be biased in patients with language impairments. Second, although these tests can quantify damage in certain cognitive areas, they often fail to reveal the specific pathophysiological mechanisms underlying cognitive dysfunction. Additionally, there is room for improvement in the sensitivity and specificity of neuropsychological tests, particularly for detecting mild cognitive impairments. Future developments should focus on enhancing test sensitivity, creating personalized assessments, and integrating modern technologies to improve accuracy.
Imaging technologies
With advancements in neuroimaging technology, imaging methods are increasingly being used to study glioma-related cognitive dysfunction. These technologies provide visual and quantitative information about brain structure and function, revealing how tumors affect brain network structures and operations.
Functional Magnetic Resonance Imaging (fMRI) is a non-invasive imaging technique that measures changes in blood oxygen level-dependent (BOLD) signals to reflect neuronal activity. fMRI is widely used to study cognitive dysfunction in glioma patients, particularly for assessing brain functions related to executive tasks, memory, language, and spatial cognition. In glioma patients, fMRI can identify changes in brain network activity associated with specific cognitive functions, illustrating the tumor’s effect on cognition. For example, studies have shown that patients with temporal lobe gliomas exhibit reduced BOLD signals during language tasks, indicating a functional impairment in that brain region. Additionally, resting-state fMRI (rs-fMRI) analyzes functional connectivity in the brain at rest, highlighting the widespread effects of gliomas on brain network structures. Silvestri E has demonstrated that glioma patients often exhibit weakened functional connectivity networks, particularly those involved in cognitive control and memory, providing new insights into the mechanisms of cognitive dysfunction [45].
Diffusion Tensor Imaging (DTI) is a specialized imaging technique used to assess the integrity of white matter fibers. DTI measures the diffusion characteristics of water molecules in white matter, revealing the directionality and integrity of white matter fibers, which reflects the efficiency of neural signal transmission. Glioma patients frequently exhibit white matter damage, especially following radiation and chemotherapy. DTI can be used to quantitatively assess the extent of this damage and its correlation with cognitive dysfunction. DTI studies have demonstrated significant white matter fiber damage in glioma patients, particularly in regions adjacent to the tumor. DTI can also reveal impaired cross-regional white matter connectivity, such as damage to the fiber tracts between the frontal and parietal lobes, which may lead to executive function and spatial cognitive impairments. By combining DTI with cognitive test results, the relationship between white matter damage and specific cognitive impairments can be more precisely elucidated, thereby offering scientific evidence for clinical interventions [46].
Despite the great potential of imaging technologies in assessing cognitive dysfunction, several challenges remain [47, 48]. First, these technologies require sophisticated equipment and procedures, which limits their accessibility to primary healthcare settings. Second, results from different imaging modalities often need to be combined with neuropsychological tests to enhance the comprehensiveness and accuracy of the assessments. Moreover, the development of imaging technologies faces technical challenges, such as improving the spatial and temporal resolution and reducing motion artefacts. Future advancements should focus on personalized assessments by integrating multimodal imaging technologies to provide a more comprehensive and accurate evaluation of cognitive dysfunction in glioma patients.
Exploration of biomarkers
In recent years, advancements in molecular biology have led to an increasing interest in the use of biomarkers to assess glioma-related cognitive dysfunction. Biomarkers can reflect neuronal damage and inflammatory responses through specific molecular indicators in the blood, cerebrospinal fluid, and other samples, providing new approaches for early diagnosis and prediction of cognitive dysfunction.
(a) Inflammatory Markers: Given the key role of neuroinflammation in glioma-related cognitive dysfunction, inflammatory markers are important indicators for assessing neuroinflammatory states. For instance, tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which are both pro-inflammatory factors, are significantly elevated in glioma patients and are closely associated with cognitive dysfunction. Detecting these inflammatory markers in the blood or cerebrospinal fluid can provide a biological basis for evaluating a patient’s cognitive status.
(b) Metabolic Markers: Changes in neural metabolism are also a critical mechanism underlying cognitive dysfunction. Metabolic markers reflect the relationship between brain energy metabolism disorders and cognitive functions. For example, changes in metabolites such as lactate and N-acetylaspartate (NAA) may indicate neuronal dysfunction. Techniques such as magnetic resonance spectroscopy (MRS) can quantitatively analyze these metabolic markers in glioma patients, revealing links between metabolic disorders and cognitive dysfunction [49].
(c) Emerging Biomarker Research Directions and Clinical Potential: Currently, biomarker research remains exploratory; however, it holds significant potential for evaluating glioma-related cognitive dysfunction. Future studies should focus on multi-biomarker joint analysis, combining assessments of inflammation, metabolism, and neuronal damage to create more accurate prediction models. Additionally, with the advancement of liquid biopsy technologies, noninvasive biomarker collection is becoming more feasible, which is expected to enhance its clinical application.
The multidimensional combination of neuropsychological tests, imaging technologies, and biomarkers provides a comprehensive tool for assessing glioma-related cognitive dysfunction. Future research should aim to integrate and innovate these technologies to advance personalized assessment and treatment, thereby addressing cognitive dysfunction in glioma patients more effectively.
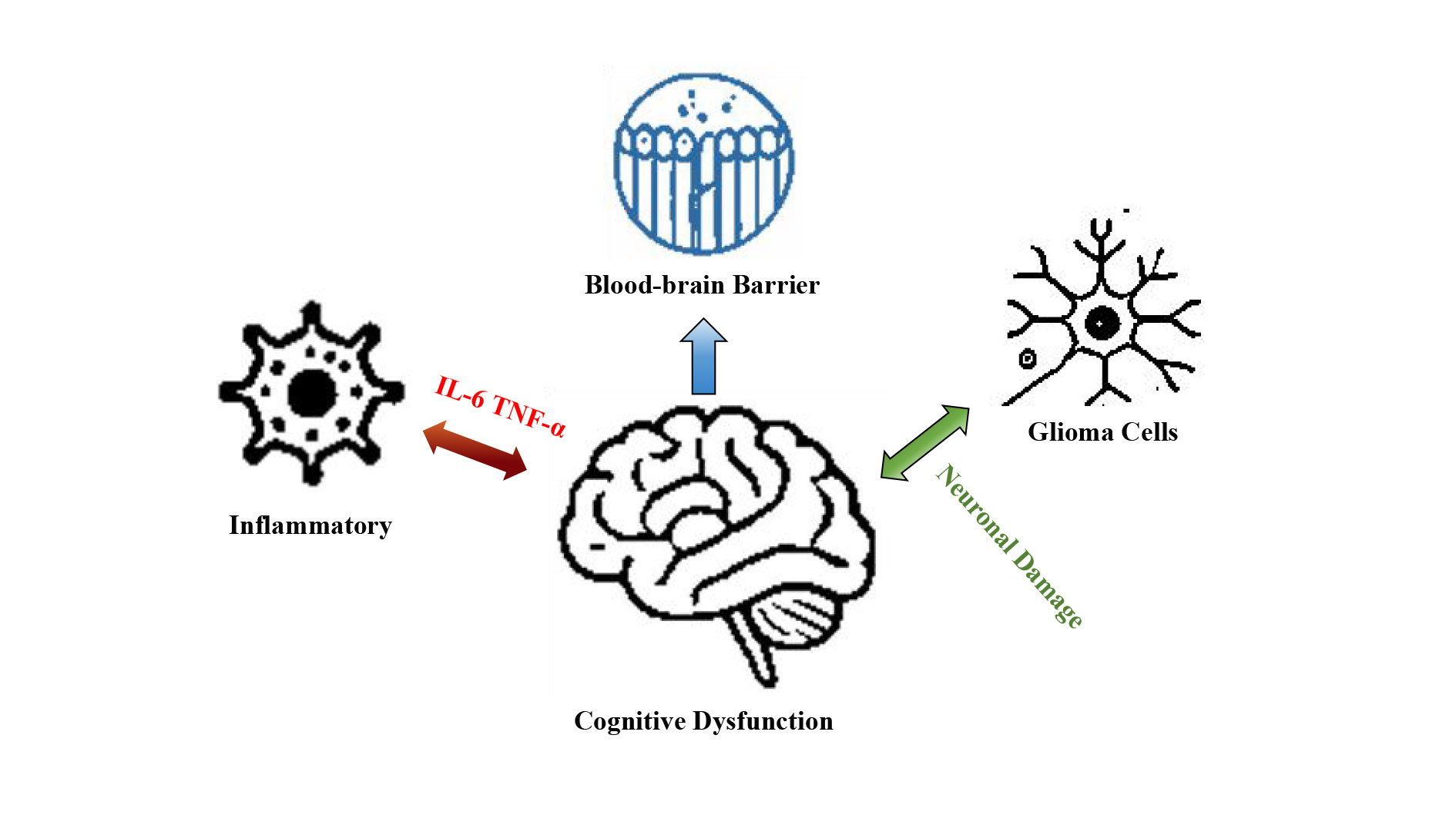 Figure 2. Glioma cells and cognitive dysfunction: the interplay of inflammatory factors and the blood-brain barrier.
Figure 2. Glioma cells and cognitive dysfunction: the interplay of inflammatory factors and the blood-brain barrier.
Pharmacological treatment
Pharmacological treatment is a primary strategy for addressing glioma-related cognitive impairment. These medications primarily work by adjusting neurotransmitter balance and mechanisms to alleviate cognitive decline.
(a) NMDA Receptor Antagonists [50]: NMDA receptor antagonists are essential drugs for treating neurocognitive disorders, with memantine being the most widely used. NMDA receptors play a crucial role in neurotransmission and synaptic plasticity. In glioma patients, NMDA receptor dysfunction often leads to excessive neuronal excitation and damage due to neuroinflammation and tumor invasion. Memantine mitigates these effects by blocking excessive NMDA receptor activation and reducing glutamate-induced neurotoxicity, thereby protecting cognitive functions. Studies have shown that memantine can improve cognitive abilities, particularly memory, attention, and executive function, in patients with glioma. However, the efficacy of memantine may vary based on individual differences and disease progression, and side effects such as headache, dizziness, and fatigue can occur.
(b) Cholinesterase Inhibitors [51, 52]: Cholinesterase inhibitors such as donepezil are commonly used to treat cognitive impairments associated with neurodegenerative diseases. These drugs inhibit the breakdown of acetylcholine, increase its concentration in the synaptic cleft, and enhance neurotransmission. Donepezil can improve cognitive function to some extent in glioma patients, particularly in those with damage to the frontal and temporal lobes. Clinical research suggests that cholinesterase inhibitors can enhance learning and memory and delay cognitive decline. However, since these drugs are primarily used for Alzheimer's disease, their application in glioma patients is still under investigation and may be accompanied by side effects, such as gastrointestinal discomfort and insomnia.
(c) Limitations and Side Effects of Pharmacological Treatment: Although pharmacological treatments have been shown to be effective in managing glioma-related cognitive impairments, their limitations are apparent. The efficacy of these drugs is often influenced by tumor progression, treatment stage, and individual physiological differences. Additionally, the long-term use of medications may lead to adaptive changes in the nervous system, potentially diminishing their effectiveness. Moreover, the side effects of these medications, such as NMDA receptor antagonists and cholinesterase inhibitors, can negatively impact the patient’s quality of life [53]. Future research should focus on optimizing drug combinations, dosages, and treatment duration to improve efficacy and reduce side effects.
Neurorehabilitation training
Neurorehabilitation training involves targeted cognitive exercises to promote the recovery and reorganization of neural functions. For glioma patients, neurorehabilitation can be tailored to address specific cognitive impairments to improve memory, attention, executive function, and language abilities.
(a) Personalized Cognitive Rehabilitation Programs: Personalized cognitive rehabilitation programs typically consist of various training modules, such as memory, language recovery, and executive function enhancement exercises. These programs often employ repetitive tasks, strategic prompts, and feedback mechanisms to activate damaged neural networks and to promote neuroplasticity. For example, memory training modules may use visual or auditory cues to enhance the encoding and recall of information, thus improving both short- and long-term memory. Executive function training focuses on task breakdown, planning, and conflict resolution to enhance attention control and problem-solving abilities [54].
(b) Clinical Effects and Current Applications of Neurorehabilitation Training: Studies have demonstrated that systematic neurorehabilitation training can significantly improve cognitive function in glioma patients, particularly in those who have undergone surgery or radiotherapy. Research indicates that patients receiving cognitive rehabilitation training show substantial improvements in executive function, memory, and language abilities, and many of these improvements are sustained over time. However, the effectiveness of neurorehabilitation training depends on several factors, including tumor type, disease stage, treatment regimen, and patient cooperation. Therefore, rehabilitation programs tailored to each patient’s specific cognitive impairments are necessary for optimal clinical outcomes [55].
Non-invasive technologies
With advancements in neuroscience, non-invasive technologies have emerged as novel interventions for cognitive impairment. These technologies improve cognitive function by externally stimulating or modulating brain activity without surgical intervention, thus offering favourable safety profiles.
(a) Transcranial Magnetic Stimulation (TMS): Transcranial magnetic stimulation (TMS) uses magnetic fields to induce electrical current in specific cortical regions. TMS has been widely used in the treatment of depression and Parkinson’s disease and can promote neuroplasticity in targeted brain areas to enhance cognitive function. TMS is primarily used to improve memory and attention in glioma patients, especially in those experiencing cognitive decline following surgery or radiotherapy. Studies have shown that TMS can improve brain connectivity and increase neuronal activity, thereby facilitating cognitive recovery. However, TMS often requires multiple sessions, and its effects may be limited in duration, necessitating further optimization of the treatment protocols.
(b) Brain-Computer Interface (BCI) Technology: Brain-computer interface (BCI) technology is an emerging neurorehabilitation tool that decodes brain activity and translates it into commands for external devices, supporting cognitive function restoration. For glioma patients, BCI technology can assist with motor recovery and cognitive functions, such as the use of virtual reality for memory and spatial cognition training. Although BCI technology is still in the early stages of clinical application, it holds promise as a potential tool for cognitive recovery with broad future applications.
(c) Advantages and Future Directions of Emerging Technologies: Non-invasive technologies offer benefits such as ease of use and good patient tolerance, making them increasingly popular in clinical practice. Future developments may focus on combining multiple non-invasive technologies, such as integrating TMS with BCI technology, to enhance treatment outcomes. Additionally, advancements in neurostimulation techniques are expected to improve the precision and personalization of these interventions, offering more effective cognitive rehabilitation options for glioma patients.
Integrated intervention strategies
Given the complex pathophysiology of glioma-related cognitive impairment, single treatment methods often fail to provide comprehensive and effective improvement. Consequently, integrated intervention strategies have become a key focus in both research and clinical practice. These strategies combine pharmacological treatment, neurorehabilitation, and non-invasive technologies to achieve more comprehensive therapeutic outcomes. The concept of multimodal intervention involves the use of different therapeutic approaches that complement each other. For example, combining pharmacological treatment with cognitive rehabilitation training can accelerate and enhance cognitive recovery. Additionally, noninvasive technologies can offer supplementary neural modulation, further boosting the effectiveness of neurorehabilitation.
Studies indicate that multimodal interventions significantly improve cognitive function in glioma patients, with those receiving integrated treatments often showing superior cognitive performance compared to patients receiving single treatments over the medium-to long-term [56-58].
Recent research on glioma-related cognitive impairments has made significant progress. In terms of pathological mechanisms, studies have progressively uncovered various ways gliomas damage brain neural networks and contribute to cognitive dysfunction, including inflammatory responses, neuronal damage, white matter fiber disruption, and neurotransmitter imbalances. Inflammatory factors and metabolic abnormalities play critical roles in both tumor growth and brain tissue damage. Concurrently, advancements in imaging technologies, such as functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI), have allowed for more precise assessments of cognitive impairments in glioma patients, and have shed light on their associations with brain structure and functional connectivity. These assessment tools provide a crucial foundation for early diagnosis and intervention.
Moreover, interventions for cognitive impairments continue to expand, with pharmacological treatments, neurorehabilitation training, and non-invasive neuromodulation technologies, such as transcranial magnetic stimulation (TMS) and brain-computer interface (BCI), which show clinical efficacy. Importantly, multimodal integrated intervention strategies that combine medications, rehabilitation training, and emerging technologies have led to improved cognitive recovery outcomes.
Challenges in clinical application
Despite significant progress in research on glioma-related cognitive impairment, several challenges remain in its clinical application. First, the complex and heterogeneous nature of the pathological mechanisms underlying cognitive impairment makes it difficult to address the diverse needs of patients using a single-treatment approach. Existing assessment tools, while partially reflective of cognitive status, are yet to achieve optimal sensitivity and specificity, particularly when evaluating microstructural changes, making precise individualized assessments difficult. Additionally, the effectiveness of pharmacological treatments is influenced by tumor type, lesion location, and individual differences, with medication side effects limiting their widespread use. Although neurorehabilitation training and non-invasive technologies show promise, their long-term effectiveness and sustainability require further validation.
Moreover, the relatively short survival time of glioma patients complicates long-term follow-up studies, and the lack of multi-center randomized controlled trials poses challenges in establishing uniform clinical guidelines based on robust evidence.
Future research directions
Future research needs to expand in several areas to address the current challenges and unresolved issues.
(a) Strengthening Mechanistic Research and Optimizing Assessment Tools: Future research should continue to explore the pathological mechanisms underlying glioma-related cognitive impairment, focusing on factors such as inflammatory responses, blood-brain barrier disruption, and neurotransmitter dysregulation. Integrating molecular biology, genetics, and multi omics technologies could help reveal individual differences among patients and identify new biomarkers for predicting and assessing the progression of cognitive impairment. Advancements in imaging technologies should aim to combine structural and functional imaging techniques to develop more precise assessment tools and to provide reliable evidence for clinical interventions.
(b) Exploring Emerging Interventions and Promoting Personalized Treatment: With the advancements in neuroscience and technology, future research should focus on exploring new interventions. Non-invasive neuromodulation technologies, such as TMS, transcranial electrical stimulation, and BCI, have significant potential for improving cognitive function. For pharmacological treatments, developing new targeted drugs that minimize side effects while enhancing efficacy should be a priority. Moreover, personalized treatment is becoming increasingly important for managing cognitive impairment. By applying multimodal interventions tailored to a patient’s pathological characteristics, cognitive assessment results, and individual differences, personalized treatment plans can become a key development direction.
(c) Emphasizing Long-Term Follow-Up and Multi-Center Clinical Research: To better validate the clinical effects and safety of various interventions, future research should emphasize long-term follow-up studies, focusing on changes in cognitive function, quality of life, and sustainability of intervention effects. Encouraging multicenter randomized controlled trials will help increase the sample sizes and improve the reliability and generalizability of the research findings. Multi-center studies can offer a comprehensive view of clinical practices across different regions and healthcare settings, thereby informing the development of more widely applicable clinical guidelines.
We would like to thank the participants of this study for sharing their experiences.
Availability of data and materials
Data and materials are available on request from the authors.
Ethical policy
Not applicable.
Author contributions
RI conceptualized, designed, conducted research, and wrote the manuscript; ZA contributed to the revision and figure production.
Competing interests
I declare that there is no conflict of interest regarding the publication of this document. I confirm that neither I nor my collaborators have any financial or personal relationships that could inappropriately influence or bias the content of this work.
Funding
None.
- Pellerino A, Caccese M, Padovan M, Cerretti G, Lombardi G: Epidemiology, risk factors, and prognostic factors of gliomas. Clin Transl Imaging 2022, 10(5): 467-475.
- Delgado-Martín B, Medina MA: Advances in the knowledge of the molecular biology of glioblastoma and its impact in patient diagnosis, stratification, and treatment. Adv Sci 2020, 7(9): 1902971.
- Cantidio FS, Gil GOB, Queiroz IN, Regalin M: Glioblastoma—treatment and obstacles. Rep Pract Oncol Radiother 2022, 27(4): 744-753.
- Fang C, Lv L, Mao S, Dong H, Liu B: Cognition deficits in Parkinson’s disease: mechanisms and treatment. Parkinsons Dis 2020, 2020(1): 2076942.
- Novak A, Vizjak K, Rakusa M: Cognitive impairment in people with epilepsy. J Clin Med 2022, 11(1): 267.
- Jia J, Xu J, Liu J, Wang Y, Wang Y, Cao Y, Guo Q, Qu Q, Wei C, Wei W: Comprehensive management of daily living activities, behavioral and psychological symptoms, and cognitive function in patients with Alzheimer's disease: a Chinese consensus on the comprehensive management of Alzheimer's disease. Neurosci Bull 2021, 37(7): 1025-1038.
- Lv K, Cao X, Wang R, Du P, Fu J, Geng D, Zhang J: Neuroplasticity of glioma patients: brain structure and topological network. Front Neurol 2022, 13: 871613.
- Seidel S, Wehner T, Miller D, Wellmer J, Schlegel U, Grönheit W: Brain tumor related epilepsy: pathophysiological approaches and rational management of antiseizure medication. Neurol Res Pract 2022, 4(1): 45.
- Pichaivel M, Anbumani G, Theivendren P, Gopal M: An overview of brain tumor. Brain Tumors 2022, 1: 1-10.
- You G, Sha Z, Jiang T: Clinical diagnosis and perioperative management of glioma-related epilepsy. Front Oncol 2021, 10: 550353.
- Hills KE, Kostarelos K, Wykes RC: Converging mechanisms of epileptogenesis and their insight in glioblastoma. Front Mol Neurosci 2022, 15: 903115.
- Mo F, Pellerino A, Soffietti R, Rudà R: Blood–brain barrier in brain tumors: biology and clinical relevance. Int J Mol Sci 2021, 22(23): 12654.
- Fernandez HR, Varma A, Flowers SA, Rebeck GW: Cancer chemotherapy related cognitive impairment and the impact of the Alzheimer’s disease risk factor APOE. Cancers 2020, 12(12): 3842.
- Krishna S, Hervey-Jumper SL: Neural Regulation of Cancer: Cancer‐Induced Remodeling of the Central Nervous System. Adv Biol 2022, 6(9): 2200047.
- Shi S, Chen T, Zhao M: The crosstalk between neurons and glia in methamphetamine-induced neuroinflammation. Neurochem Res 2022, 47(4): 872-884.
- Jayaram MA, Phillips JJ: Role of the microenvironment in glioma pathogenesis. Annu Rev Pathol 2024, 19(1): 181-201.
- Poonaki E, Kahlert UD, Meuth SG, Gorji A: The role of the ZEB1–neuroinflammation axis in CNS disorders. J Neuroinflammation 2022, 19(1): 275.
- Rodríguez-Gómez JA, Kavanagh E, Engskog-Vlachos P, Engskog MKR, Herrera AJ, Espinosa-Oliva AM, Joseph B, Hajji N, Venero JL, Burguillos MA: Microglia: agents of the CNS pro-inflammatory response. Cells 2020, 9(7): 1717.
- Raffaele S, Lombardi M, Verderio C, Fumagalli M: TNF production and release from microglia via extracellular vesicles: impact on brain functions. Cells 2020, 9(10): 2145.
- Bernaus A, Blanco S, Sevilla A: Glia crosstalk in neuroinflammatory diseases. Front Cell Neurosci 2020, 14: 209.
- Babkina II, Sergeeva SP, Gorbacheva LR: The role of NF-κB in neuroinflammation. Neurochem J 2021, 15(2): 114-128.
- Alfeo F, Lanciano T, Abbatantuono C, Gintili G, De Caro MF, Curci A, Taurisano P: Cognitive, Emotional, and Daily Functioning Domains Involved in Decision-Making among Patients with Mild Cognitive Impairment: A Systematic Review. Brain Sci 2024, 14(3): 278.
- Dutta M, Murray LL, Stark BC: Assessing the integrity of executive functioning in chronic aphasia. Aphasiology 2023, 37(6): 869-906.
- van Oostveen WM, de Lange ECM: Imaging techniques in Alzheimer’s disease: a review of applications in early diagnosis and longitudinal monitoring. Int J Mol Sci 2021, 22(4): 2110.
- Podwalski P, Szczygieł K, Tyburski E, Sagan L, Misiak B, Samochowiec J: Magnetic resonance diffusion tensor imaging in psychiatry: a narrative review of its potential role in diagnosis. Pharmacol Rep 2021, 73(1): 43-56.
- Coelho A, Fernandes HM, Magalhães R, Moreira PS, Marques P, Soares JM, Sousa N: Signatures of white-matter microstructure degradation during aging and its association with cognitive status. Sci Rep 2021, 11(1): 4517.
- Zhao YH, Xu Y: Effect of comprehensive nursing based on cognitive behavior on psychological function of glioma patients. Neuropsychiatr Dis Treat 2021, 17: 777-785.
- Thenuwara G, Curtin J, Tian F: Advances in diagnostic tools and therapeutic approaches for gliomas: A comprehensive review. Sensors 2023, 23(24): 9842.
- You G, Sha Z, Jiang T: Clinical diagnosis and perioperative management of glioma-related epilepsy. Front Oncol 2021, 10: 550353.
- Lange F, Hörnschemeyer J, Kirschstein T: Glutamatergic mechanisms in glioblastoma and tumor-associated epilepsy. Cells 2021, 10(5): 1226.
- Menon V, D’Esposito M: The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 2022, 47(1): 90-103.
- Jia S, Yang H, Huang F, Fan W: Systemic inflammation, neuroinflammation and perioperative neurocognitive disorders. Inflamm Res 2023, 72(9): 1895-1907.
- Winter SF, Dietrich J: Neurological complications of radiation. In: Oxford Textbook of Neurohaematology. 2024: 143.
- Ramírez-Guerrero S, Vargas-Cuellar MP, Charry-Sánchez JD, Talero-Gutiérrez C: Cognitive sequelae of radiotherapy in primary brain tumors. Interdiscip Neurosurg 2021, 26: 101305.
- Mo F, Pellerino A, Soffietti R, Rudà R: Blood–brain barrier in brain tumors: biology and clinical relevance. Int J Mol Sci 2021, 22(23): 12654.
- Laha D, Grant R, Mishra P, Nilubol N: The role of tumor necrosis factor in manipulating the immunological response of tumor microenvironment. Front Immunol 2021, 12: 656908.
- Nengroo MA, Verma A, Datta D: Cytokine chemokine network in tumor microenvironment: Impact on CSC properties and therapeutic applications. Cytokine 2022, 156: 155916.
- Borst K, Dumas AA, Prinz M: Microglia: Immune and non-immune functions. Immunity 2021, 54(10): 2194-2208.
- Duffau H: White matter tracts and diffuse lower-grade gliomas: the pivotal role of myelin plasticity in the tumor pathogenesis, infiltration patterns, functional consequences and therapeutic management. Front Oncol 2022, 12: 855587.
- Krishna S, Kakaizada S, Almeida N, Brang D, Hervey-Jumper S: Central nervous system plasticity influences language and cognitive recovery in adult glioma. Neurosurgery 2021, 89(4): 539-548.
- Chen Z, Ye N, Teng C, Li X: Alternations and applications of the structural and functional connectome in gliomas: a mini-review. Front Neurosci 2022, 16: 856808.
- Hamer RP, Yeo TT: Current status of neuromodulation-induced cortical prehabilitation and considerations for treatment pathways in lower-grade glioma surgery. Life 2022, 12(4): 466.
- Maas DA, Douw L: Multiscale network neuroscience in neuro-oncology: How tumors, brain networks, and behavior connect across scales. Neuro Oncol Pract 2023, 10(6): 506-517.
- Mazrooyisebdani M, Nair VA, Garcia-Ramos C, Mohanty R, Meyerand E, Hermann B, Ahmed R: Graph theory analysis of functional connectivity combined with machine learning approaches demonstrates widespread network differences and predicts clinical variables in temporal lobe epilepsy. Brain Connect 2020, 10(1): 39-50.
- Silvestri E, Moretto M, Facchini S, Castellaro M, Anglani M, Monai E, Corbetta M: Widespread cortical functional disconnection in gliomas: an individual network mapping approach. Brain Commun 2022, 4(2): fcac082.
- Hu AM, Ma YL, Li YX, Han ZZ, Yan N, Zhang YM: Association between changes in white matter microstructure and cognitive impairment in white matter lesions. Brain Sci 2022, 12(4): 482.
- Piau A, Wild K, Mattek N, Kaye J: Current state of digital biomarker technologies for real-life, home-based monitoring of cognitive function for mild cognitive impairment to mild Alzheimer disease and implications for clinical care: systematic review. J Med Internet Res 2019, 21(8): e12785.
- Zhuang L, Yang Y, Gao J: Cognitive assessment tools for mild cognitive impairment screening. J Neurol 2021, 268(5): 1615-1622.
- Le Page LM, Guglielmetti C, Taglang C, Chaumeil MM: Imaging brain metabolism using hyperpolarized 13C magnetic resonance spectroscopy. Trends Neurosci 2020, 43(5): 343-354.
- Kikuchi T: Is memantine effective as an NMDA receptor antagonist in adjunctive therapy for schizophrenia? Biomolecules 2020, 10(8): 1134.
- Walczak-Nowicka ŁJ, Herbet M: Acetylcholinesterase inhibitors in the treatment of neurodegenerative diseases and the role of acetylcholinesterase in their pathogenesis. Int J Mol Sci 2021, 22(17): 9290.
- Lista S, Vergallo A, Teipel SJ, Lemercier P, Giorgi FS, Gabelle A, Garaci F, Mercuri NB, Babiloni C, Gaire BP et al: Determinants of approved acetylcholinesterase inhibitor response outcomes in Alzheimer’s disease: relevance for precision medicine in neurodegenerative diseases. Ageing Res Rev 2023, 84: 101819.
- Balázs N, Bereczki D, Kovács T: Cholinesterase inhibitors and memantine for the treatment of Alzheimer and non-Alzheimer dementias. Ideggyogy Sz 2021, 74(11-12): 379-87.
- Zelazo PD: Executive function and psychopathology: A neurodevelopmental perspective. Annu Rev Clin Psychol 2020, 16(1): 431-454.
- Ziegler DA, Anguera JA, Gallen CL, Hsu WY, Wais PE, Gazzaley A: Leveraging technology to personalize cognitive enhancement methods in aging. Nat Aging 2022, 2(6): 475-483.
- Noll K, King AL, Dirven L, Armstrong TS, Taphoorn MJ, Wefel JS: Neurocognition and health-related quality of life among patients with brain tumors. Hematol Oncol Clin North Am 2022, 36(1): 269-282.
- Zeng XX, Zeng J, Zhu BY: Future generation of combined multimodal approach to treat brain glioblastoma multiforme and potential impact on micturition control. Rev Neurosci 2022, 33(3): 313-326.
- Fouda MA, Kallman S, Boorstin R, Sacks-Zimmerman A, Pannullo SC, Bender HA: The unseen impact–a deep dive into neurocognitive impairment among patients with intracranial meningiomas: a comprehensive systematic review of the literature. Neurosurg Rev 2024, 47(1): 294.
Asia-Pacific Journal of Oncology
print ISSN: 2708-7980, online ISSN: 2708-7999
 Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript