Review | Open Access
Applications and future prospects of artificial intelligence in the diagnosis and prognosis of lung cancer
Soha Kareem1
1Department of Anesthesia, University of Edinburgh Medical School, Scotland, United Kingdom.
Correspondence: Soha Kareem (Department of Anesthesia, University of Edinburgh Medical School, Scotland, United Kingdom; E-mail: gossipmahi@gmail.com).
Asia-Pacific Journal of Oncology 2025, 6: 47-54. https://doi.org/10.32948/ajo.2025.05.25
Received: 25 May 2025 | Accepted: 28 Aug 2025 | Published online: 15 Sep 2025
Key words artificial intelligence, diagnosis, lung cancer, machine learning, treatment
Despite the implementation of numerous diagnostic and interventional approaches for lung malignancy in clinical settings, the prognosis of patients afflicted with this malignancy remains unfavorable [12]. At present, the principal diagnostic approach for lung tumor predominantly depends on computed tomography (CT) [13] and the scanning and histological examination of tissue samples [14, 15]. CT scans are prone to misdiagnosis and tissue biopsies are invasive procedures. Concurrently, there remains a pressing need to enhance the sensitivity and specificity of non-invasive markers for lung carcinoma detection [16, 17]. The diagnostic challenges associated with lung cancer are compounded by several factors, including the anatomical site of the tumor, histological classification, presence of metastatic disease, and associated complications [18]. Consequently, a significant proportion of individuals diagnosed with lung cancer exhibit metastases at the time of initial presentation [19]. Surgical intervention coupled with chemotherapeutic regimens is the predominant therapeutic approach for pulmonary carcinoma is surgical intervention coupled with chemotherapeutic regimens [20]. NSCLC, which accounts for 85% of all lung cancer cases, has a higher chance of success with early surgical removal than small cell lung cancer, which has the highest degree of malignancy and tends to recur following surgical intervention [21]. Radiation therapy and chemotherapy are more effective in treating small cell lung cancers [22].
AI is an emerging area of research that focuses on formulating theories, devising methodologies, crafting technologies, and designing application systems that aim to mimic, enhance, and broaden the scope of human cognitive abilities [23, 24]. The technical architecture of AI encompasses four principal components: natural language processing, visual processing, human-computer interaction, and ML [25]. Empirical evidence suggests that rule-based AI frameworks have shown variable degrees of clinical utility in pulmonary malignancies, encompassing aspects such as diagnosis [26], treatment [27], and prognosis [28]. Figure 1 illustrates the generic structure of an AI workflow for pulmonary cancer detection and management. This review examines the latest advancements in AI applications and their clinical implementation in lung cancer research and clinical practice.
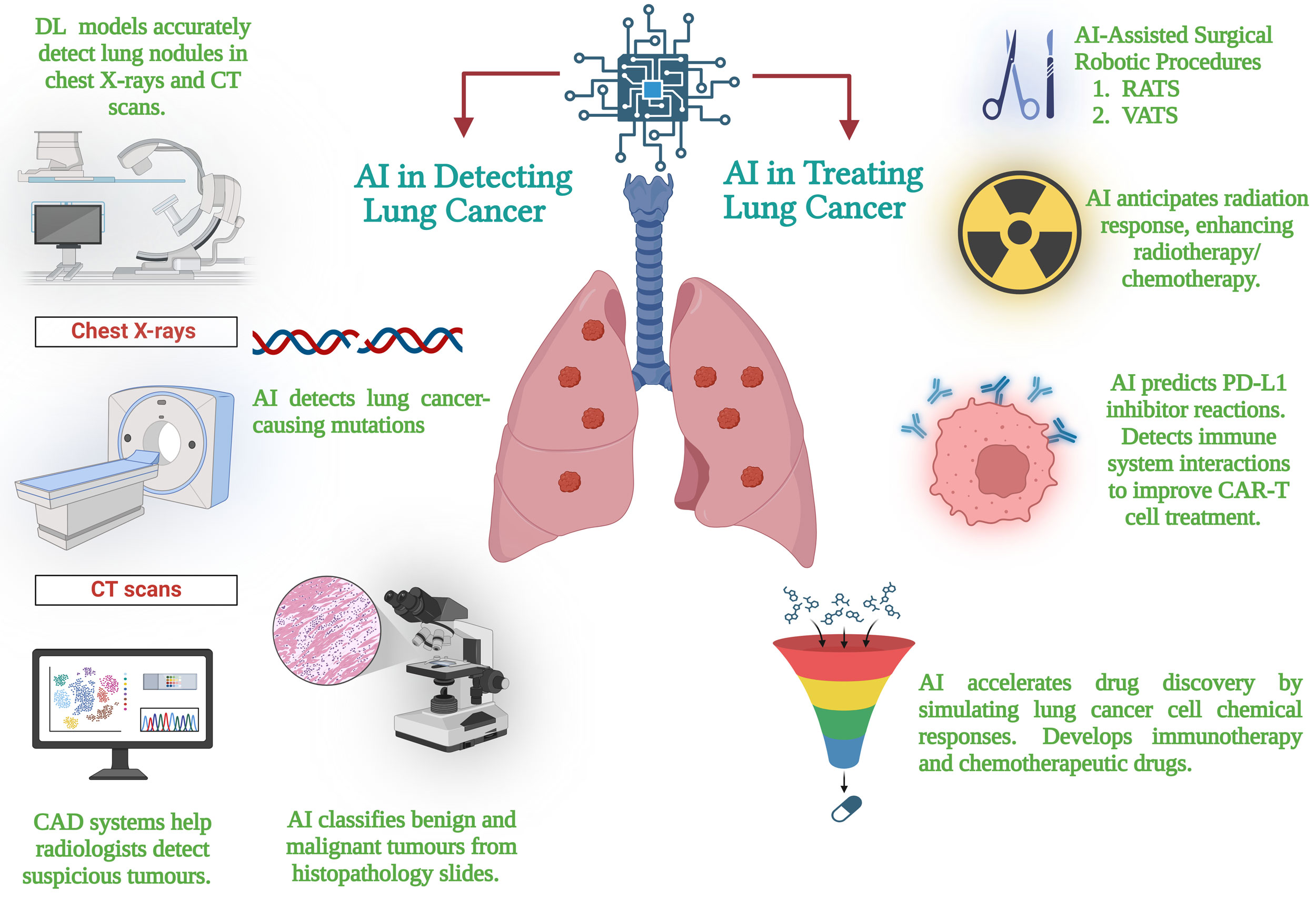 Figure 1. The general outline of an AI workflow for the diagnosis and treatment of lung cancer. The figure illustrates innovations in early diagnosis facilitated by AI via imaging evaluation, specific therapy enabled by genome sequencing profiling, escalated drug discovery, and enhanced surveillance of patients for better outcome and recurrence prediction.
Figure 1. The general outline of an AI workflow for the diagnosis and treatment of lung cancer. The figure illustrates innovations in early diagnosis facilitated by AI via imaging evaluation, specific therapy enabled by genome sequencing profiling, escalated drug discovery, and enhanced surveillance of patients for better outcome and recurrence prediction.
Kehl et al.'s study utilized a natural language processing (NLP) model to predict cancer development. This study used a significant collection comprising more than 300,000 imaging results obtained from 16,780 patients. With a concordance index of 0.76 and an area under the curve (AUC) of 0.77, the researchers effectively created a model to estimate patient prognosis and therapy adjustments. Thus, they proposed using this technique to instantly identify suitable applicants for particular clinical investigations [29].
The presence of pulmonary nodules is strongly associated with the initial stage of lung carcinoma. These nodules are predominantly identified using thoracic computed tomography (CT). In CT scans, nodules manifest as spherical or irregular opacities, which may present with clear or indistinct borders and measure up to 3 cm in their maximal dimension [30, 31]. Although most pulmonary lesions are non-malignant, some may evolve into lung neoplasms. Research conducted through the National Lung Screening Trial illustrated the efficacy of lung cancer screening in reducing mortality rates within populations at elevated risk [32]. The expanded use of pulmonary cancer diagnostic methodologies will invariably result in the detection of numerous pulmonary lesions with indeterminate malignant characteristics. The proliferation of lung cancer surveillance protocols will invariably result in the detection of numerous pulmonary lesions with indeterminate malignant potential.
The process of detecting pulmonary nodules presents significant challenges, with radiologists exhibiting a marked variability in their diagnostic proficiency. This discrepancy in detection sensitivity is attributable to the complex interplay of nodule characteristics, including their dimensions, morphology, spatial distribution, radiodensity, and contextual relationships with adjacent anatomical structures [33]. Extensive manual interpretation of medical images can result in an overlooked diagnosis. Currently, there is heightened interest in leveraging AI for the identification of pulmonary nodules. Within the realm of lung cancer screening, AI's potential extends beyond automated detection, encompassing patient stratification and optimization of low-dose CT scan reconstruction processes [34].
Khosravan et al. developed S4ND, an innovative deep-learning-based approach for lung nodule detection. This method utilizes a unified three-dimensional (3D) convolutional neural network (CNN) architecture with dense connectivity that undergoes end-to-end training for a complete detection workflow. The described methodology exhibits 95.2% sensitivity in lung nodule detection through a single network's feedforward pass [35]. Following the identification of lung nodules, the subsequent phase involved differentiation from the encompassing pulmonary parenchyma. The U-Net CNN developed by Ronneberg et al. enhances the process of biomedical image segmentation. This sophisticated method accurately defined the pulmonary nodule boundaries at the pixel level. This level of detail in characterizing the nodule lays the groundwork for future quantitative analysis and tracking of its development over time [36].
Bhattacharyya et al. developed a differentiable binarization network (DB-NET), which is a weighted bidirectional feature network-based U-NET architecture. The segmentation of pulmonary nodules was greatly improved using this novel method. This methodology resulted in improved performance in the segmentation of ground-glass, cavitary, and small nodules [37]. Table 1 outlines a detailed review of AI implementation in the domain of lung nodule analysis. AI techniques have several distinct advantages and limitations that must be considered. The k-medoid clustering algorithm is robust in the presence of interference and irregularities; however, it is computationally demanding and vulnerable to preliminary parameter choices. Conversely, CNN are used in image-processing tasks; however, they require substantial labeled datasets and considerable computational resources to achieve optimal performance. Although 3D CNNs can capture higher levels of spatial data in medical images, they incur higher processing costs. Although Dense Net enhances the information flow through dense connections, it also increases the model complexity. Although suitable for medical picture segmentation, the 3D U-NET requires longer training cycles.
AI's role in the pathogenesis of lung cancer
Lung cancer is primarily classified into two categories: NSCLC and SCLC. Among these, NSCLC is the most prevalent form, constituting approximately 80%-85% of all documented lung cancer cases [43]. Empirical evidence has established the ability of AI to accurately differentiate lung cancer subtypes and to forecast clinical progression in patients with NSCLC. Yu et al. conducted an extensive extraction of image features using histopathology whole-slide images of lung adenocarcinoma and squamous cell carcinoma patients obtained from The Cancer Genome Atlas (TCGA). The investigators employed regularized ML techniques to identify the most crucial variables for discriminating between patients with short-term and long-term survival outcomes. The results showed that robotically separated imaging features can predict the prognosis of patients with lung cancer, thereby improving the discipline of precision oncology. This analytical method can be used for histopathological imaging of various organs [44].
In a related analysis, Coudray et al. randomly retrieved 1,634 histology slides drawn from the TCGA data repository using a powerful CNN technique (Inception v3). Their goal was to classify these images according to morphological traits into normal lung tissue, squamous cell carcinoma, or lung adenocarcinoma. The results showed a remarkable AUC of 0.97, demonstrating a high degree of conformity with the pathologists' evaluations [45].
The diagnosis site for lung cancers has been transformed by the application of AI systems in the examination of cytopathological specimens acquired by fine-needle aspiration [46]. These sophisticated computing techniques help to investigate several biological samples like phlegm, bronchial lavage fluid, pulmonary mucus, and needle aspirates [47]. Using AI-driven approaches helps physicians distinguish lung cancer from other respiratory conditions, hence improving the accuracy and efficiency of pulmonary medicine diagnosis processes. In an analysis by Gonzalez et al., a DL algorithm was developed using cytology and biopsies acquired from forty individuals. Based on structural features, this CNN-based method seeks to differentiate SCLC from large-cell neuroendocrine carcinoma. Although the study had a small sample size, the applied DL models showed amazing performance in accurately spotting most cases for both cancer types [48].
Different cell populations' spatial arrangement can help to clarify the dynamics of neoplastic cells, their interactions within the tumor microenvironment, and the immunological defense systems of the host. Wang et al. presented ConvPath, a sophisticated computational tool for lung cancer computer-based histological image analysis. This unique program uses CNNs to handle several tasks like nuclear segmentation, tumor cell classification, stromal cell analysis, lymphocyte analysis, and tumor environment-related feature analysis from lung tumor histo-morphological images [49]. Moreover, this method helps pathogenic images to be spatial representations of lymphocytes, stromal cells, and tumor cells. Such change could greatly improve the research of cellular spatial configurations and their impact on tumor development and metastatic processes.
AI's use in identifying gene alterations in lung cancer
Recent developments in computer science allow researchers to investigate vast lung cancer genotyping datasets using cutting-edge ML and DL techniques [50]. These innovative techniques have enabled the research of genetic modifications linked to the beginning and spread of lung cancer (Figure 2). Moreover, these analytical instruments have been useful in pointing up potential driver mutations that might be important in the evolution of lung cancer. A sequence of genetic changes known as driver mutations controls lung cancer development. These fundamental genetic changes include gene mutations such as epidermal growth factor receptor (EGFR) [51], Kirsten rat sarcoma viral oncogene homolog (KRAS) [52], and anaplastic lymphoma kinase (ALK) fusion proteins [53]. Notably, these genetic aberrations serve as potential therapeutic targets for the management of lung adenocarcinoma.
Genetic mutation information was extracted from TCGA and correlated with the corresponding patient samples. A CNN was constructed to analyze the ten most prevalent genetic mutations in lung adenocarcinoma. The analysis demonstrated that pathological images could be utilized to predict six genes Serine/Threonine Kinase 11 (STK11), EGFR, FAT Atypical Cadherin 1 (FAT1), SETBP1 (SET Binding Protein 1), KRAS, and Tumor Protein p53 (TP53), yielding AUC values between 0.733 and 0.856 [45]. In a separate investigation, Wang et al. engineered an advanced AI framework, termed the Fully Automated Artificial Intelligence System (FAIS). This cutting-edge computational model was designed in order to accurately predict EGFR mutations in lung tumors and analyze progression-free survival (PFS) of individuals undergoing EGFR-targeted therapies. The implementation of noninvasive CT imaging in conjunction with FAIS yielded remarkable predictive outcomes for EGFR gene mutations, with AUC values ranging from 0.748 to 0.813. Furthermore, the system revealed a statistically significant correlation with PFS (log-rank p<0.05). Especially, these results were acquired without manual annotation, highlighting how FAIS might transform individualized treatment plans and lung cancer diagnosis.
Along with the more common EGFR and KRAS mutations, ALK fusions in NSCLC represent another genetic abnormality. Song et al. designed and verified an enhanced DL algorithm for ALK fusion status in NSCLC in a thorough study. From a cohort of 937 NSCLC patients, the researchers examined CT scans and clinical pathology data to get an area under the curve of 0.8046. This study also looked at the clinical results of 91 patients using ALK tyrosine kinase inhibitor (TKI) treatment. The results exposed a notable difference in PFS between participants who were positive and negative for ALK. More specifically, whereas other patients showed a median PFS of 7.5 months (P = 0.010), ALK-positive patients showed a median PFS of 16.8 months [54].
For lung cancer, AI offers great promise in identifying genetic abnormalities. AI-driven algorithms can quickly spot DNA changes, create individualized treatment regimens, and project patient reactions to treatment. These features minimize needless use of medical resources by improving diagnosis accuracy and treatment effectiveness. It is believed that the synthesis of several data repositories would help AI systems clarify fresh treatment targets and increase the precision of medical implementation.
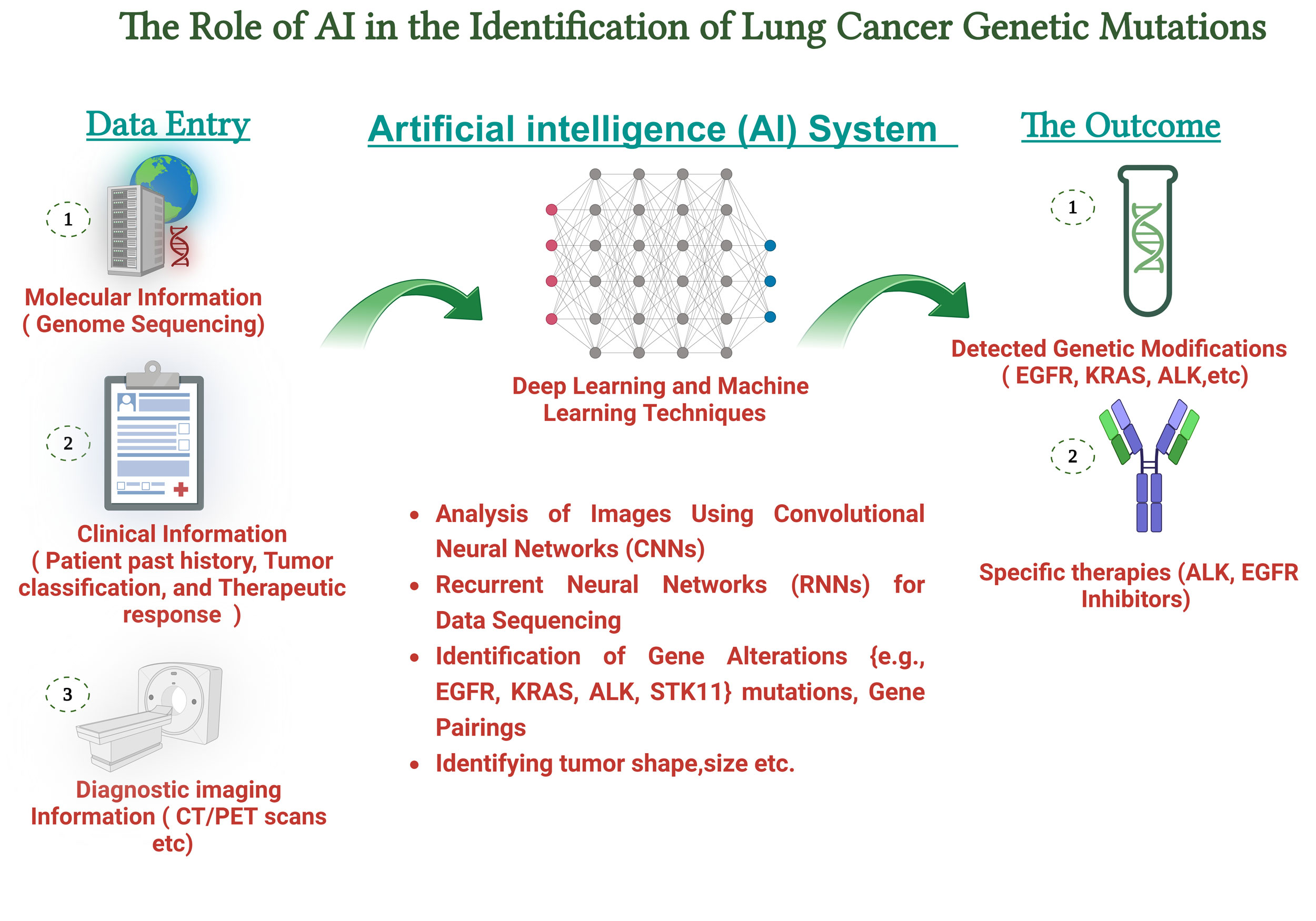 Figure 2. The AI workflow combines gene sequencing information, bioinformatics evaluations, and neural network models to pinpoint significant mutations, including EGFR, ALK, KRAS, and STK11. The process encompasses data preparation, extraction of features, and model prediction to improve the precision of mutation classification.
Figure 2. The AI workflow combines gene sequencing information, bioinformatics evaluations, and neural network models to pinpoint significant mutations, including EGFR, ALK, KRAS, and STK11. The process encompasses data preparation, extraction of features, and model prediction to improve the precision of mutation classification.
|
Table 1. Summarizing the applications of AI in lung nodule detection, procedures, and findings. |
|||||
|
Year |
Objective |
Dataset |
Procedures |
Findings |
Ref |
|
2019 |
Lung nodule categorization |
Out of 289 benign cases, 74 were identified during training, and 50 were identified during validation |
Clustering with k-medoids and theory of information |
AUC was 0.965, with a sensitivity of 100%; Specificity is 96% |
[38] |
|
2020 |
Identifying and categorizing lung nodules |
1018 Lung Image Database Consortium and Image Database Resource Initiative (LIDC-IDRI) Cases |
3D CNN |
Sensitivity: 95.6% |
[39] |
|
2021 |
To distinguish benign and malignant GGNs |
513 Ground-glass nodules (GGNs) |
Deep neural network, or 3D U-NET |
Accuracy: 75.6% |
[40] |
|
2022 |
Detection of lung nodules |
888 CT scans were performed |
Sphere center-points matching SCPM-Net |
The average sensitivity was 89.2% |
[41] |
|
2023 |
Segmenting and extracting features from pulmonary nodules |
1,186 nodules |
Support vector machine (SVM); Random Forest (RF); Support vector regression (SVR) |
Total precision: 75% |
[42] |
Clinical settings have seen a significant increase in the use of 3D reconstruction techniques, which are essential components of AI-augmented surgical procedures [55]. The application of sophisticated computational methods for 3D image reconstruction, utilizing various imaging modalities including CT, MRI, and PET-CT, facilitates the generation of improved and easily accessible 3D representations of respiratory abnormalities and their adjacent anatomical organs [56, 57]. This advanced visualization technique significantly enhances the precision and effectiveness of preoperative planning. The implementation of this advanced technology instills greater confidence in medical practitioners and offers crucial support for surgical planning and its execution. Studies have shown that approximately 20-30% of individuals exhibit variations in their pulmonary vascular anatomy [58]. Recognition of anatomical variations is of paramount importance in lung cancer surgery, particularly for effective preoperative planning and precise intraoperative navigation of the bronchoscope. A retrospective cohort analysis demonstrated that cardiothoracic surgeons exhibited 85% precision in recognizing anatomical abnormalities using AI-augmented CT imaging. This process requires a median duration of 2 min, with intervals ranging from 1 to 3 min [59]. The application of AI in reconstructive techniques facilitates surgeons' ability to swiftly and precisely identify anatomical patterns, thereby enhancing the efficacy of preoperative planning for segmentectomy procedures.
The development of 3D lung reconstruction models is aided by semi-automated software applications, such as Mimics, OsiriX, and 3DSlicer [60, 61]. These sophisticated applications allow for the simulation of anatomical features, assist in the delineation of pulmonary segments, and support the precise localization of lesions within the respiratory system. Surgical outcomes are markedly enhanced through the utilization of intraoperative navigation techniques that employ precise 3D reconstruction models, resulting in reduced operative duration and elevated rates of procedural success [62]. However, the widespread use of these systems in clinical practice may be hindered by the substantial time investment and high level of skill required. To improve thoracic surgery and evaluate its precision, effectiveness, and safety for clinical applications, Li et al. developed a completely automatic 3D reconstruction tool using 3D CNNs. The AI system significantly reduced the duration of the procedure, reducing the lobectomy by 24.5 minutes (P < 0.001) and the segmentectomy by 20 minutes (P = 0.007). The AI system outperformed the manual restoration software (Mimics) in terms of model quality scores (P < 0.001) and reduced the model rebuilding period by 14.2 min (P < 0.001) [63].
Currently, AI-driven 3D reconstruction technologies primarily emphasize the autonomous reconstruction of the pulmonary vasculature and bronchial structures [64]. To date, there is a paucity of research on automated 3D reconstruction systems for tissue morphological alterations following preoperative treatment, which represents a potential avenue for future advancements in AI in the domain of pulmonary cancer 3D modeling. The constant advancement of AI technology has led to significant transformations in medical practice [65]. In the foreseeable future, the field may witness the emergence of sophisticated AI-driven devices, including robotic platforms for surgical procedures, AI-augmented systems for lung cancer biopsy and therapy, and autonomous robotic units capable of executing pulmonary oncological operations.
AI-powered presurgical analysis
Three principal modalities are commonly employed in lung cancer therapeutics: operative excision, radiotherapeutic interventions, and chemotherapy [66]. The field of clinical practice has witnessed a remarkable evolution in recent years, driven by substantial progress in molecular biology and genomics research. Targeted therapies, immunological treatments, neoadjuvant regimens, and new therapeutic modalities—among other therapeutic approaches—have been widely adopted as a consequence in part to these scientific developments [67]. The three-part combination of Tumour Node Metastasis (TNM) staging, immunohistochemical markers, and histological classification defines the basis of lung cancer therapy choice [68]. These fundamental elements serve as primary determinants for guiding therapeutic decisions in patients with pulmonary malignancies.
In clinical practice, determining the invasiveness of lung cancer prior to surgery remains challenging because the true extent of pathological invasion can only be ascertained through a thorough examination of the surgical specimens [69]. In a study by Onozato et al., 873 patients who underwent segmental resections or lobectomy for primary bronchogenic cancer were investigated. From pre-surgical PET and CT scans, the researchers collected quantitative imaging features and assessed seven ML algorithms and an ensemble model (ENS) combining the PET and CT data. All models demonstrated high performance in predicting tumour invasiveness, achieving an AUC of 0.880 or higher in the training set. When tested on an independent dataset, the ENS model outperformed the others, exhibiting the greatest average AUC of 0.880 and a precision of 0.804 [70].
To investigate the stratification of lung cancer risk, Zhou et al. proposed a sophisticated computational model known as the ensemble multi-view 3D Convolutional Neural Network (EMV-3D-CNN). With an accuracy of 77.6%, their model surpassed that of experienced physicians in the risk categorization of invasive adenocarcinoma. This provides comprehensive predictive histological data [71]. The research team led by Lv et al. introduced a DL approach that exhibited efficacy comparable to that of an intra-operative frozen segment examination in evaluating tumor thickness. This creative approach could help clinical decisions on the suitable level of surgical excision [72].
Future usage of AI in lung cancer research and treatment is likely to be much more extensive and have more important impact. AI is becoming more adept in helping medical professionals precisely identify lung issues during imaging diagnostics as computer algorithms develop and training datasets grow. AI allows real-time monitoring and enhancement of the therapy efficacy in lung cancer patients by merging sensor networks with tracking information. Because of developments in robotic surgery and virtual reality technologies, surgical techniques supported by AI have now become available. This sophisticated approach enables surgeons to operate with more precision, therefore reducing the possibility of intraoperative problems and post-operative adverse outcomes.
Ultimately, as computing capability, medical knowledge, and relevant data keep developing, AI will become increasingly more important in each aspect of lung cancer. This will create opportunities for future customized treatment methods, better diagnostic procedures, and higher screening accuracy. Though AI is a helpful tool, it will not replace doctors; rather, it will enhance their knowledge and abilities. The medical sector has to find ways to use the synergies between AI and healthcare professionals if we are to better manage lung cancer and provide more efficient patient-centered treatment.
No applicable.
Ethics approval
No applicable.
Data availability
The data will be available upon request.
Funding
None.
Authors’ contribution
Soha Kareem contributed to the conception, design and writing of this review article.
Competing interests
The authors declare no competing interests.
- Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, Mariotto AB, Lowy DR, Feuer EJ: The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med 2020, 383(7): 640-649.
- Bade BC, Dela Cruz CS: Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med 2020, 41(1): 1-24.
- Choi HK, Mazzone PJ: Lung Cancer Screening. Med Clin North Am 2022, 106(6): 1041-1053.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71(3): 209-249.
- Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 2020, 70(1): 7-30.
- Osarogiagbon RU, Van Schil P, Giroux DJ, Lim E, Putora PM, Lievens Y, Cardillo G, Kim HK, Rocco G, Bille A, et al: The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Overview of Challenges and Opportunities in Revising the Nodal Classification of Lung Cancer. J Thorac Oncol 2023, 18(4): 410-418.
- Raso MG, Bota-Rabassedas N, Wistuba, II: Pathology and Classification of SCLC. Cancers (Basel) 2021, 13(4): 820.
- Travis WD: Lung Cancer Pathology: Current Concepts. Clin Chest Med 2020, 41(1): 67-85.
- Choi S, Cho SI, Ma M, Park S, Pereira S, Aum BJ, Shin S, Paeng K, Yoo D, Jung W, et al: Artificial intelligence-powered programmed death ligand 1 analyser reduces interobserver variation in tumour proportion score for non-small cell lung cancer with better prediction of immunotherapy response. Eur J Cancer 2022, 170: 17-26.
- Travis WD, Dacic S, Wistuba I, Sholl L, Adusumilli P, Bubendorf L, Bunn P, Cascone T, Chaft J, Chen G, et al: IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J Thorac Oncol 2020, 15(5): 709-740.
- Rami-Porta R, Bolejack V, Giroux DJ, Chansky K, Crowley J, Asamura H, Goldstraw P: The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014, 9(11): 1618-1624.
- Horeweg N, Scholten ET, de Jong PA, van der Aalst CM, Weenink C, Lammers JW, Nackaerts K, Vliegenthart R, ten Haaf K, Yousaf-Khan UA, et al: Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014, 15(12): 1342-1350.
- Kim J, Dabiri B, Hammer MM: Micronodular lung disease on high-resolution CT: patterns and differential diagnosis. Clin Radiol 2021, 76(6): 399-406.
- Ren F, Fei Q, Qiu K, Zhang Y, Zhang H, Sun L: Liquid biopsy techniques and lung cancer: diagnosis, monitoring and evaluation. J Exp Clin Cancer Res 2024, 43(1): 96.
- Borczuk AC, Shah L, Pearson GD, Walter KL, Wang L, Austin JH, Friedman RA, Powell CA: Molecular signatures in biopsy specimens of lung cancer. Am J Respir Crit Care Med 2004, 170(2): 167-174.
- Zhao W, Yang J, Sun Y, Li C, Wu W, Jin L, Yang Z, Ni B, Gao P, Wang P, et al: 3D Deep Learning from CT Scans Predicts Tumor Invasiveness of Subcentimeter Pulmonary Adenocarcinomas. Cancer Res 2018, 78(24): 6881-6889.
- Ding Y, Feng H, Yang Y, Holmes J, Liu Z, Liu D, Wong WW, Yu NY, Sio TT, Schild SE, et al: Deep-learning based fast and accurate 3D CT deformable image registration in lung cancer. Med Phys 2023, 50(11): 6864-6880.
- Salgia R: Diagnostic challenges in non-small-cell lung cancer: an integrated medicine approach. Future Oncol 2015, 11(3): 489-500.
- Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, Harris LJ, Detterbeck FC: Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143(5 Suppl): e211S-e250S.
- Jedlička V: Surgical treatment of lung cancer. Klin Onkol 2021, 34(Supplementum 1): 35-42.
- Mogavero A, Bironzo P, Righi L, Merlini A, Benso F, Novello S, Passiglia F: Deciphering Lung Adenocarcinoma Heterogeneity: An Overview of Pathological and Clinical Features of Rare Subtypes. Life (Basel) 2023, 13(6): 1291.
- Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, Dacic S, Jain D, Kerr KM, Lantuejoul S, et al: The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022, 17(3): 362-387.
- Christie JR, Lang P, Zelko LM, Palma DA, Abdelrazek M, Mattonen SA: Artificial Intelligence in Lung Cancer: Bridging the Gap Between Computational Power and Clinical Decision-Making. Can Assoc Radiol J 2021, 72(1): 86-97.
- Zhang C, Xu J, Tang R, Yang J, Wang W, Yu X, Shi S: Novel research and future prospects of artificial intelligence in cancer diagnosis and treatment. J Hematol Oncol 2023, 16(1): 114.
- Rajpurkar P, Chen E, Banerjee O, Topol EJ: AI in health and medicine. Nat Med 2022, 28(1): 31-38.
- Duan X, Yang Y, Tan S, Wang S, Feng X, Cui L, Feng F, Yu S, Wang W, Wu Y: Application of artificial neural network model combined with four biomarkers in auxiliary diagnosis of lung cancer. Med Biol Eng Comput 2017, 55(8): 1239-1248.
- Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts H: Artificial intelligence in radiology. Nat Rev Cancer 2018, 18(8): 500-510.
- Hart GR, Roffman DA, Decker R, Deng J: A multi-parameterized artificial neural network for lung cancer risk prediction. PLoS One 2018, 13(10): e0205264.
- Kehl KL, Groha S, Lepisto EM, Elmarakeby H, Lindsay J, Gusev A, Van Allen EM, Hassett MJ, Schrag D: Clinical Inflection Point Detection on the Basis of EHR Data to Identify Clinical Trial-Ready Patients With Cancer. JCO Clin Cancer Inform 2021, 5: 622-630.
- Bankier AA, MacMahon H, Colby T, Gevenois PA, Goo JM, Leung ANC, Lynch DA, Schaefer-Prokop CM, Tomiyama N, Travis WD, et al: Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2024, 310(2): e232558.
- Khan A, Tariq I, Khan H, Khan SU, He N, Zhiyang L, Raza F: Lung Cancer Nodules Detection via an Adaptive Boosting Algorithm Based on Self-Normalized Multiview Convolutional Neural Network. J Oncol 2022, 2022: 5682451.
- Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD: Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011, 365(5): 395-409.
- MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, Mehta AC, Ohno Y, Powell CA, Prokop M, et al: Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284(1): 228-243.
- Cellina M, Cacioppa LM, Cè M, Chiarpenello V, Costa M, Vincenzo Z, Pais D, Bausano MV, Rossini N, Bruno A, et al: Artificial Intelligence in Lung Cancer Screening: The Future Is Now. Cancers (Basel) 2023, 15(17): 4344.
- Khosravan N, Bagci U: S4ND: Single-shot single-scale lung nodule detection. In: Medical Image Computing and Computer Assisted Intervention–MICCAI 2018: 21st International Conference, Granada, Spain, September 16-20, 2018, Proceedings, Part II 11: 2018: Springer; 2018: 794-802.
- Ronneberger O, Fischer P, Brox T: U-net: Convolutional networks for biomedical image segmentation. In: Medical image computing and computer-assisted intervention–MICCAI 2015: 18th international conference, Munich, Germany, October 5-9, 2015, proceedings, part III 18: 2015: Springer; 2015: 234-241.
- Bhattacharyya D, Thirupathi Rao N, Joshua ESN, Hu YC: A bi-directional deep learning architecture for lung nodule semantic segmentation. Vis Comput 2022, 39: 5245–5261.
- Uthoff J, Stephens MJ, Newell JD, Jr., Hoffman EA, Larson J, Koehn N, De Stefano FA, Lusk CM, Wenzlaff AS, Watza D, et al: Machine learning approach for distinguishing malignant and benign lung nodules utilizing standardized perinodular parenchymal features from CT. Med Phys 2019, 46(7): 3207-3216.
- Naqi SM, Sharif M, Jaffar A: Lung nodule detection and classification based on geometric fit in parametric form and deep learning. Neural Comput Appl 2020, 32(9): 4629-4647.
- Hu X, Gong J, Zhou W, Li H, Wang S, Wei M, Peng W, Gu Y: Computer-aided diagnosis of ground glass pulmonary nodule by fusing deep learning and radiomics features. Phys Med Biol 2021, 66(6): 065015.
- Luo X, Song T, Wang G, Chen J, Chen Y, Li K, Metaxas DN, Zhang S: SCPM-Net: An anchor-free 3D lung nodule detection network using sphere representation and center points matching. Med Image Anal 2022, 75: 102287.
- Tang TW, Lin WY, Liang JD, Li KM: Artificial intelligence aided diagnosis of pulmonary nodules segmentation and feature extraction. Clin Radiol 2023, 78(6): 437-443.
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA: Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008, 83(5): 584-594.
- Yu KH, Zhang C, Berry GJ, Altman RB, Ré C, Rubin DL, Snyder M: Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat Commun 2016, 7: 12474.
- Coudray N, Ocampo PS, Sakellaropoulos T, Narula N, Snuderl M, Fenyö D, Moreira AL, Razavian N, Tsirigos A: Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med 2018, 24(10): 1559-1567.
- Shafi S, Parwani AV: Artificial intelligence in diagnostic pathology. Diagn Pathol 2023, 18(1): 109.
- Lotter W, Hassett MJ, Schultz N, Kehl KL, Van Allen EM, Cerami E: Artificial Intelligence in Oncology: Current Landscape, Challenges, and Future Directions. Cancer Discov 2024, 14(5): 711-726.
- Gonzalez D, Dietz RL, Pantanowitz L: Feasibility of a deep learning algorithm to distinguish large cell neuroendocrine from small cell lung carcinoma in cytology specimens. Cytopathology 2020, 31(5): 426-431.
- Wang S, Wang T, Yang L, Yang DM, Fujimoto J, Yi F, Luo X, Yang Y, Yao B, Lin S, et al: ConvPath: A software tool for lung adenocarcinoma digital pathological image analysis aided by a convolutional neural network. EBioMedicine 2019, 50: 103-110.
- Zhang B, Shi H, Wang H: Machine Learning and AI in Cancer Prognosis, Prediction, and Treatment Selection: A Critical Approach. J Multidiscip Healthc 2023, 16: 1779-1791.
- da Cunha Santos G, Shepherd FA, Tsao MS: EGFR mutations and lung cancer. Annu Rev Pathol 2011, 6: 49-69.
- Karachaliou N, Mayo C, Costa C, Magrí I, Gimenez-Capitan A, Molina-Vila MA, Rosell R: KRAS mutations in lung cancer. Clin Lung Cancer 2013, 14(3): 205-214.
- Won JK, Keam B, Koh J, Cho HJ, Jeon YK, Kim TM, Lee SH, Lee DS, Kim DW, Chung DH: Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol 2015, 26(2): 348-354.
- Song Z, Liu T, Shi L, Yu Z, Shen Q, Xu M, Huang Z, Cai Z, Wang W, Xu C, et al: The deep learning model combining CT image and clinicopathological information for predicting ALK fusion status and response to ALK-TKI therapy in non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging 2021, 48(2): 361-371.
- Sadeghi AH, Mank Q, Tuzcu AS, Hofman J, Siregar S, Maat A, Mottrie A, Kluin J, De Backer P: Artificial intelligence-assisted augmented reality robotic lung surgery: Navigating the future of thoracic surgery. JTCVS Tech 2024, 26: 121-125.
- Sadeghi AH, Maat A, Taverne Y, Cornelissen R, Dingemans AC, Bogers A, Mahtab EAF: Virtual reality and artificial intelligence for 3-dimensional planning of lung segmentectomies. JTCVS Tech 2021, 7: 309-321.
- Manafi-Farid R, Askari E, Shiri I, Pirich C, Asadi M, Khateri M, Zaidi H, Beheshti M: [(18)F]FDG-PET/CT Radiomics and Artificial Intelligence in Lung Cancer: Technical Aspects and Potential Clinical Applications. Semin Nucl Med 2022, 52(6): 759-780.
- Smelt JLC, Suri T, Valencia O, Jahangiri M, Rhode K, Nair A, Bille A: Operative Planning in Thoracic Surgery: A Pilot Study Comparing Imaging Techniques and Three-Dimensional Printing. Ann Thorac Surg 2019, 107(2): 401-406.
- Chen X, Wang Z, Qi Q, Zhang K, Sui X, Wang X, Weng W, Wang S, Zhao H, Sun C, et al: A fully automated noncontrast CT 3-D reconstruction algorithm enabled accurate anatomical demonstration for lung segmentectomy. Thorac Cancer 2022, 13(6): 795-803.
- Cheng GZ, San Jose Estepar R, Folch E, Onieva J, Gangadharan S, Majid A: Three-dimensional Printing and 3D Slicer: Powerful Tools in Understanding and Treating Structural Lung Disease. Chest 2016, 149(5): 1136-1142.
- Nemec SF, Molinari F, Dufresne V, Gosset N, Silva M, Bankier AA: Comparison of four software packages for CT lung volumetry in healthy individuals. Eur Radiol 2015, 25(6): 1588-1597.
- Wu Z, Huang Z, Qin Y, Jiao W: Progress in three-dimensional computed tomography reconstruction in anatomic pulmonary segmentectomy. Thorac Cancer 2022, 13(13): 1881-1887.
- Li X, Zhang S, Luo X, Gao G, Luo X, Wang S, Li S, Zhao D, Wang Y, Cui X, et al: Accuracy and efficiency of an artificial intelligence-based pulmonary broncho-vascular three-dimensional reconstruction system supporting thoracic surgery: retrospective and prospective validation study. EBioMedicine 2023, 87: 104422.
- Chen X, Xu H, Qi Q, Sun C, Jin J, Zhao H, Wang X, Weng W, Wang S, Sui X, et al: AI-based chest CT semantic segmentation algorithm enables semi-automated lung cancer surgery planning by recognizing anatomical variants of pulmonary vessels. Front Oncol 2022, 12: 1021084.
- Johnson M, Albizri A, Simsek S: Artificial intelligence in healthcare operations to enhance treatment outcomes: a framework to predict lung cancer prognosis. Ann Oper Res 2022, 308(1): 275-305.
- Li Y, Yan B, He S: Advances and challenges in the treatment of lung cancer. Biomed Pharmacother 2023, 169: 115891.
- Passaro A, Brahmer J, Antonia S, Mok T, Peters S: Managing Resistance to Immune Checkpoint Inhibitors in Lung Cancer: Treatment and Novel Strategies. J Clin Oncol 2022, 40(6): 598-610.
- Kawamoto N, Tsutani Y, Kamigaichi A, Ohsawa M, Mimae T, Miyata Y, Okada M: Tumour location predicts occult N1 nodal metastasis in clinical stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2023, 63(2): ezac575.
- Montagne F, Guisier F, Venissac N, Baste JM: The Role of Surgery in Lung Cancer Treatment: Present Indications and Future Perspectives-State of the Art. Cancers (Basel) 2021, 13(15): 3711.
- Onozato Y, Iwata T, Uematsu Y, Shimizu D, Yamamoto T, Matsui Y, Ogawa K, Kuyama J, Sakairi Y, Kawakami E, et al: Predicting pathological highly invasive lung cancer from preoperative [(18)F]FDG PET/CT with multiple machine learning models. Eur J Nucl Med Mol Imaging 2023, 50(3): 715-726.
- Zhou J, Hu B, Feng W, Zhang Z, Fu X, Shao H, Wang H, Jin L, Ai S, Ji Y: An ensemble deep learning model for risk stratification of invasive lung adenocarcinoma using thin-slice CT. NPJ Digit Med 2023, 6(1): 119.
- Lv Y, Wei Y, Xu K, Zhang X, Hua R, Huang J, Li M, Tang C, Yang L, Liu B, et al: 3D deep learning versus the current methods for predicting tumor invasiveness of lung adenocarcinoma based on high-resolution computed tomography images. Front Oncol 2022, 12: 995870.
Asia-Pacific Journal of Oncology
print ISSN: 2708-7980, online ISSN: 2708-7999
 Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript